Introduction
“The single biggest threat to man’s continued dominance on the planet is the virus.”
Joshua Lederberg, Nobel Laureate[1]
As a new virus, SARS-CoV-2, races through the world infecting millions, killing hundreds of thousands and fundamentally changing the way people live, Lederberg’s words seem omniscient.
How did the world get here?
About 550 gigatons of carbon (Gt C) biomass is distributed across life on Earth, and humans, with only a 0.06 Gt C share, are a minuscule minority in the biosphere.[2] Yet, over a relatively short period of human history, the human species has transformed, and continues to transform, Earth’s climate, natural resources, and biodiversity to such a degree that the International Union of Geological Societies (IUGS) has accepted a new age in Earth’s geologic history – the Anthropocene or “the age of humans”.[3] According to the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES), humans have “significantly altered” three-fourths of Earth’s land and two-thirds of its oceans.[4]
Agriculture, domestication of livestock, and the Industrial Revolution have resulted in the expansion of human and livestock populations, with major ecological outcomes including the destruction of wild animal habitats. This has increased the frequency of human-animal contacts, and with it the threat of new diseases emerging into human populations. Increased human activity has complex links to deforestation, loss of habitat and biodiversity, and has heightened the prevalence and risk of zoonoses.[a]
Indeed, animal spillovers account for two-thirds of all human infectious diseases and three-fourths of newly emerging diseases.[5] This can happen directly through animal bites, the consumption of raw or undercooked meat or other animal products, or through contaminated water. Indirect spillover results when humans come into contact with infected animals or through vectors such as mosquitoes and ticks. Both wild and domesticated animals can pass disease to humans.[6]
Zoonoses, Spillover and Impact
A 2008 study of emerging infectious disease (EID) events between 1940 and 2004 found these to be distributed non-randomly across the globe.[7] It found EIDs to be dominated by pathogens that emerge in wild animals and then transfer to human populations to cause disease, and that such events were increasing over time. The report showed correlations with socio-economic, environmental and ecological factors, predicted emerging disease ‘hotspots’, and recommended increased surveillance. However, little happened in these regions, including in India, which was then already being predicted to be a prominent hotspot.
Viruses comprise only 14 percent of known human pathogens but 44 percent of new and emerging pathogens.[8] Some of the biggest public health threats of the 20th century came from viruses such as influenza and HIV, both of which spilled from animals into humans. The “Spanish Flu” of 1918-19 infected an estimated 500 million people, then comprising one-third of the world’s population, with up to 50 million deaths,[9] of which about 12-18 million were reported in India.[10]Decades later, the “Asian Flu” of 1957-58[11] and the “Hong Kong” flu of 1968-69[12] together killed another two to four million people. Since the ongoing HIV/AIDS pandemic began in the 1980s, about 75 million people have become infected, and over 32 million have died.[13]
Swine Flu emerged in 2009 as the first pandemic of the 21st century, followed a decade later by the current COVID-19, caused by SARS-CoV-2. At the time of writing, COVID-19 has spread to 215 countries, causing almost 16 million confirmed cases and over 639,000 deaths.[14] There have been other viral epidemics this century, which due to their limited geographic spread did not become pandemics: SARS coronavirus (2002-03); MERS coronavirus (2012-ongoing); and Ebola virus (2013-16). The pace of emergence of new viruses threatening human health has continuously increased over the past 25 years. Of the 20 diseases considered by the World Health Organization (WHO) to have the potential to develop into future pandemics, 16 have viruses as the causative agents.[15]
Researchers at the University of California at Davis have shown the spillover potential to be greatest for viruses from domestic animals and species that are better adapted to human landscapes, and from wild animals whose habitats have been exploited or destroyed.[16] Birds and wild mammals have many potentially zoonotic viruses, and more frequent animal-human contacts increase the spillover probability. Anthropogenic activities and environmental change remain the biggest drivers for the spillover of zoonoses.[17]
Every new zoonotic disease starts with humans coming into contact with wildlife. Climate change shapes the biogeographical distribution of species, as well as the location and frequency of these meetings. A 2017 review in the journal Sciencelooked at 40,000 species globally and found around half to be already on the move as a result of warming temperatures and changing rainfall patterns.[18] In general, species are seeking cooler temperatures by moving towards the Earth’s poles at an average rate of 10 miles per decade for land animals, and 45 miles per decade for marine animals.[19] When terrestrial animals move, they also bring along their viruses and spread them. If the new species move into areas where humans are prevalent, new opportunities emerge for pandemics to evolve.
Besides influenza viruses that have a complex human-animal-bird ecology,[20]two dominant links emerge when one considers all viruses that have emerged in the past 50 years: bats and mosquitoes. Both of these are impacted by changes to ecology and climate, and become drivers of disease spillover into humans.
Bats, other mammals and their viruses
Bats harbour many viruses, which can be transmitted either directly or indirectly into humans. Examples of bat-borne viruses that have caused epidemics include the SARS, MERS, Ebola, Nipah Hendra, and SARS-CoV-2.[21] Of the 60 viral species reported to be associated with bats, 59 are RNA viruses responsible for emerging and re-emerging human infectious diseases.[22] Bats are ubiquitous, have large population sizes and species diversity, which together with their close habitation to humans and livestock make them important sources of zoonoses.[23] About 117 species of bats have been reported in India, but little is known about the viruses they carry.[24]
Loss of forests and changed land use for agriculture and human settlement present major risks for disease spillover. Examples include the outbreaks due to Nipah virus in Malaysia (1998-99), Siliguri (2001), and Kerala (2018) in India and in Bangladesh (2001-14). Fruit-eating bats affected by deforestation roosted on trees and in barns, from where secretions and half-eaten fruits transmitted the virus to pigs and other domestic animals, before transferring to humans.[25]Similarly, transmission of Hendra virus was observed from bats to horses, and then to humans.[26] The Rabies virus and others in that family such as Australian Bat Lyssavirus (ABLV), Lago virus or Duvenhage virus can be directly transmitted via bite to humans.[27] Though direct transmission remains rare, it stresses the risk associated with high biodiversity of bats and the increasing proximity of bat and human populations.
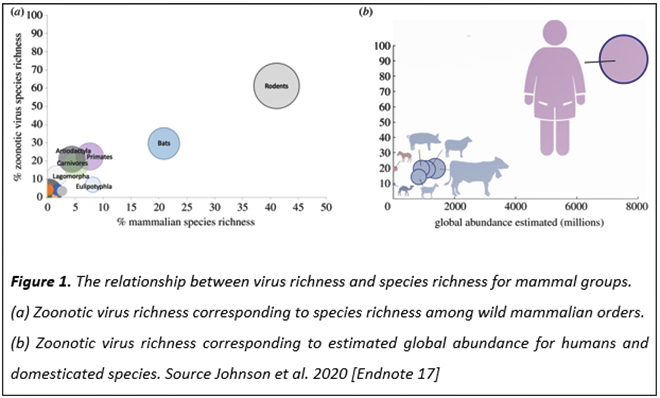
A modelling study has compared virus richness and species richness for various mammal groups, and the abundance of various mammals on Earth.[28] Rodents comprise about 45 percent of mammals, and carry 60 percent of zoonotic virus species; bats follow with over 20 percent mammalian species and 30 percent of zoonotic virus species (See Figure 1). Humans are the most abundant species on Earth and are susceptible to over 80 percent of known zoonotic viruses. Humans provide the biggest target for zoonotic viruses that have spilled over from rodents, bats and other mammals, and will continue to be so in the future.[29]
Changing ecology can drive bats and other animals into new areas, and with them carry their viruses and other zoonoses. A 2018 study found climate change to enhance the risk of spillover of the Hendra virus that can pass from flying foxes (a species of bats) to humans through horses.[30] Rapid population growth, climate change, and high rates of greenhouse gas (GHG) emissions are also predicted to expand areas prone to Ebola epidemics by 20 to 30 percent.[31] It is estimated that by 2070, Ebola could break out, on average, once every 10 years, from the current average of 17 years.[32] For a disease with extremely high case fatality rates,[b] this should be a matter of great concern.
Mosquito-borne pathogens and global warming
Of around 3,500 mosquito species on Earth, those belonging to the Anophelesand Aedes genera[c] are the most prominent vectors of disease transmission in humans. The Anopheles genus has about 460 species, of which only about 30-40 can transmit the malaria parasite Plasmodium. The Aedes genus has about 700 species, which were originally present only in tropical and subtropical zones, but can now be found on all continents except Antarctica. Within this genus, two species – Ae aegypti and Ae albopictus are particularly adept at transmitting viruses that cause dengue, yellow fever, chikungunya and Zika.
With global warming, the mosquito season extends past the summer months in temperate regions of the world. Does this influence the risk of being infected with mosquito-borne pathogens?
In 2012 a dengue outbreak occurred in Madeira, Portugal—the first in Europe since the 1920s. Using historical and projected temperatures from 1901 to 2099, and the vectorial capacity of Aedes mosquitoes, a multi-country research team made estimates of the dengue epidemic potential in Europe.[33] They discovered a slow increase in the intensity and duration of dengue transmission over the 20thcentury, and more rapid increases projected in the 21st century; the rate of increase was correlated with the levels of GHG emissions.[34] Thus, besides vector control, mitigating these emissions would crucially reduce the future epidemic potential of dengue in Europe and other temperate areas of the world.
Indeed, temperature is considered to be an important driver of mosquito-borne viral disease outbreaks due to its broad effects on the mosquito life cycle, biting rates, and the rate at which mosquitoes acquire and transmit viruses. Researchers at Stanford University in the US and elsewhere modeled how rising temperatures might influence the behaviour of Ae aegypti and Ae albopictus mosquitoes and their disease transmission risk around the world.[35] They also used field data on three human diseases transmitted by these mosquitoes – chikungunya, dengue and Zika. The results showed transmission to take place between 18 to 34°C, with maximum transmission at 26 to 29°C. The models also indicated extended seasonal or year-round transmission in tropical and subtropical regions, and transmission in temperate areas to be limited to up to three summer months per year.[36]
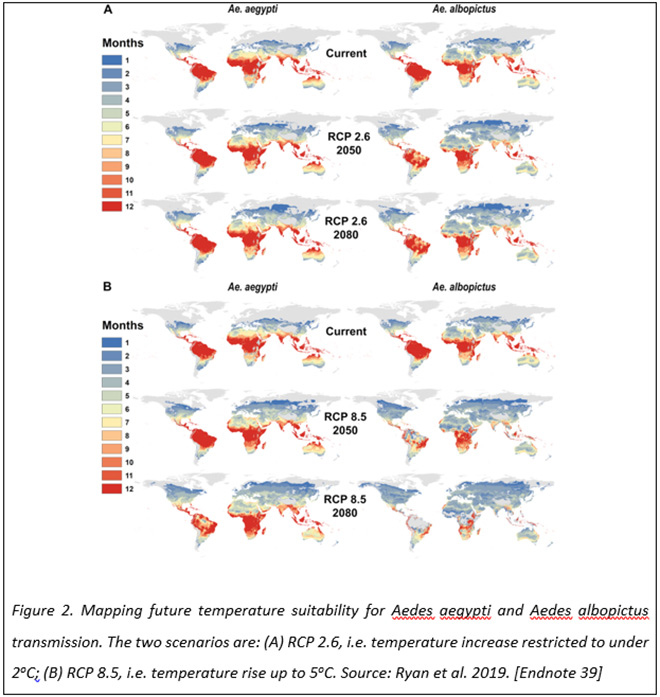
The Paris Agreement on Climate Change is an international agreement that sets out a global framework to avoid dangerous climate change by limiting global warming to well below 2°C.[37] Using this and a scenario with no climate action where global warming could reach 5oC, a group of US researchers modeled how the geographic range, the seasonality of disease risk, and the overall number of people exposed might change for the Ae aegypti and Ae albopictus mosquitoes.[38] The results show that if no climate mitigation is carried out, almost one billion people, a majority being in Europe, could face their first exposure to mosquito-borne diseases by 2080 (Figure 2). Limiting the warming to below 2oC may halve the number of people at risk for diseases such as dengue fever, Zika and chikungunya.[39]
The Lancet Countdown[d] 2019 Report concludes that the climate has become progressively more conducive for the transmission of many diseases, putting increasing numbers of people at risk.[40] For example, climate change is increasing people’s susceptibility to malaria by broadening the geographic range and lengthening the reproductive season of its Anopheles mosquito vector. This is also true for viral diseases that are spread by Aedes mosquitoes. Lyme disease, which is spread by ticks, is also increasing its range and seasonality in many parts of North America and Europe. At the same time, waterborne diseases such as cholera and cryptosporidiosis are increasing with more frequent droughts and flooding.[41]
When the ice and permafrost melt
The September 2019 Report of the Intergovernmental Panel on Climate Change (IPCC) warns that if warming continues at the current rate, a large part of the permafrost[e] could melt by 2100, releasing large amounts of greenhouse gases.[42] It is estimated that about 1.7 trillion tonnes of carbon is locked into the permafrost, mainly as methane and carbon dioxide. Thawing would convert this carbon sink into an emitter, which would further speed up global warming.[43] As the ice thaws and permafrost melts, there is the possibility of re-emergence of dormant human pathogens, which have been locked away in glaciers and permafrost for hundreds or thousands of years. There is already growing evidence of this.
In January 2020, researchers reported finding several viruses from two ice core samples from a glacier in northwestern Tibet, dating back 520 years and 15,000 years. Of the 33 virus genera found in the ice, 28 were novel and had never been seen before.[44] Scientists also discovered three different “giant” viruses[f] from a 30,000-year-old Siberian permafrost. Named as Pandoraviruses, Pithovirus sibericum and Mollivirus sibericum, two of these could easily be revived in the laboratory into their infectious state.[45],[46] Though these viruses infect only algae, the discovery underlines a concern that unknown animal or human pathogens may also be lurking in environments that would become more accessible with global warming.
This is not restricted to viruses. In 2016 in Siberia, a 12-year-old boy died and at least 115 people were hospitalised due to an outbreak of anthrax, a bacterial disease, which is believed to have spread from the carcass of a reindeer buried in 1941 and uncovered that summer due to high temperatures. It is thought that reindeer were first infected after coming into contact with the remains, and subsequently spread the disease to humans.[47] Ultraviolet light, oxygen and high temperature are bad for most microbes, whereas they thrive in cold, dark and no-oxygen environments. That makes permafrost, ice cores and deep ocean sediments excellent breeding grounds for microbes.
A virus re-emerging in an isolated region of Siberia or from under a Tibetan glacier might have a low risk of exposure compared to one that reaches the urban areas with high density of people. But that may change as humans and animals respond to rising temperatures. The thawing of ice in the poles may open new navigation routes, and a changing climate may also expand the range of species to new parts of the world. The climate is changing fast and the effects are uncertain. It is therefore prudent to keep these ecosystems on our radar as potential sites for disease emergence.
Epilogue
“The deviation of man from the state in which he was originally placed by nature seems to have proved to him a prolific source of diseases.”
Edward Jenner, 1798
Pandemics appear for a reason. And that reason is the size of the host population and the degree to which it has disturbed its environment. Upsetting the balance of nature is something the human species has done rather well. The exploitation of wildlife has not only increased the risk of virus transmission to people, but has led to the decline of many wildlife species, putting them at risk of extinction. Human encroachment into wildlife habitats has similarly resulted in increased contact with wild animals and heightened the rates of virus spillover. Climate change has also driven viral reservoirs and vectors into newer areas of human habitation, further increasing the risk of spillover. Besides other environmental impacts, increasing global temperatures also carry the risk of unleashing viruses that have been dormant for thousands of years.
The devastation wrought by the COVID-19 pandemic has shown us that business-as-usual is not always necessary. When people return to normal after the pandemic, the imperative is to find safe and sustainable ways to co-exist with wildlife and reduce human footprint on the shared environment. Human beings are the dominant species on Earth, causing the alteration of ecosystems. Ultimately, nature will have the last word. This pandemic is a warning for worse that lies ahead. It is also an opportunity to set the balance right.
About the Author
Dr. Shahid Jameel established and for 25 years led the Virology Group at the International Centre for Genetic Engineering and Biotechnology, New Delhi. He is currently CEO of the DBT/Wellcome Trust India Alliance, a biomedical research public charity based in India.
Endnotes
[a] Zoonoses are diseases or infections that are naturally transmissible from vertebrate animals to humans.
[b] The average Ebola case fatality rate is around 50%. Case fatality rates have varied from 25% to 90% in past outbreaks. Source: World Health Organization
[c] The taxonomic rank between family and species
[d] A scientific collaboration between 35 leading academic centres and UN agencies across the world.
[e] Frozen soil found mostly in the Northern Hemisphere, where it covers about 25% of exposed land. It is considered to be thousands of years old.
[f] “Giant” viruses have sizes (0.6–1.5 µmeter) and genome length (0.6–2.8 Megabase), while most commonly known viruses have much smaller sizes (0.02–0.4 µmeter) and genome length (0.002–0.3 Megabase); 1 µmeter = 1 millionth of a meter; 1 Megabase = 1 million bases.
[1] Joshua Lederberg. Speech before the Irvington Institute for Medical Research, Bankers Trust Company, New York, February 8, 1994.
[2] Yinon M. Bar-On, Rob Phillips, and Ron Milo, “The biomass distribution on Earth,” Proc Natl Acad Sci (USA) 115, 2018: 6506-11. DOI: 10.1073/pnas.1711842115.
[3] “Subcommission on Quaternary Startigraphy”, Working Group on the Anthropocene, Accessed July 6, 2020,
[4] “Global Assessment Report on Biodiversity and Ecosystem Services”, Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, Accessed July 6, 2020.
[5] Ibid
[6] Centers for Disease Control and Prevention, USA Accessed July 6, 2020.
[7] Kate E. Jones, Nikkita G. Patel, Marc A. Levy, Adam Storeygard, Deborah Balk, John L. Gittleman and Peter Daszak, “Global trends in emerging infectious diseases,” Nature 451, 2008: 990-3. doi:10.1038/nature06536
[8] Mark Woolhouse, Fiona Scott, Zoe Hudson, Richard Howey, and Margo Chase-Topping, “Human viruses: discovery and emergence,” Philos Trans R Soc Lond B Biol Sci. 367, 2012: 2864-71. doi:10.1098/rstb.2011.0354
[9] Centers for Disease Control and Prevention, USA, Accessed 24 July, 2020.
[10] David Arnold, “Death and the Modern Empire: the 1918-19 Influenza Epidemic in India,” Trans RHS. 29, 2019: 181-200.
[11] Centers for Disease Control and Prevention, USA, Accessed 24 July, 2020.
[12] Centers for Disease Control and Prevention, USA, Accessed 24 July, 2020.
[13] United Nations Programme on HIV/AIDS, Accessed 24 July, 2020.
[14] Worldometer, Accessed July 24, 2020.
[15] World Health Organization, Accessed July 6, 2020.
[16] Ibid
[17] Christine K. Johnson, Peta L. Hitchens, Pranav S. Pandit, Julie Rushmore, Tierra Smiley Evans, Cristin CW Young and Megan M Doyle, “Global shifts in mammalian population trends reveal key predictors of virus spillover risk”, Proc R Soc B. 287, 2020:20192736.
[18] Ibid
[19] Gretta T. Pecl, Miguel B. Araujo, Johann D. Bell, et al, “Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being,” Science 355, 2017: eaai9214. doi:10.1126/science.aai9214.
[20] Udayan Joseph, Su, Yvonne C. F., Dhanasekharan Vijaykrishna, and Gavin J. D. Smith, “The ecology and adaptive evolution of influenza A interspecies transmission,” Influenza and Other Respiratory Viruses 11, 2017: 74–84. doi: 10.1111/irv.12412
[21] Ina Smith, and Lin-Fa Wang, “Bats and their virome: an important source of emerging viruses capable of infecting humans,” Curr Opin Virol. 3, 2013: 84-91.
[22] Cara E. Brook, and Andrew P. Dobson, “Bats as ‘special’ reservoirs for emerging zoonotic pathogens,” Trends Microbiol. 23, 2015: 172-80. doi:10.1016/j.tim.2014.12.004
[23] Kevin J. Olival, Jonathan H. Epstein, Lin-Fa Wang, and Hume E. Field, “Are bats unique viral reservoirs?” in New Directions in Conservation Medicine: applied Cases of Ecological Health, eds. Alonso A. Aguirre, Richard Ostfeld, and Peter Daszak (New York: Oxford University Press, 2012), 195–212.
[24] Mohana Basu, “Bat coronavirus found in two Indian species of bats for the first time: ICMR study”, The Print, April 15, 2020.
[25] Mandeep S. Chadha, James, A. Comer, Lewis Lowe, et al, “Nipah virus-associated encephalitis outbreak, Siliguri, India,” Emerg Infect Dis. 12, 2006: 235-40. doi:10.3201/eid1202.051247
[26] Sarah Weatherman, Heinz Feldmann, and Emmie de Wit, “Transmission of henipaviruses,” Curr Opin Virol. 28, 2017: 7-11. doi: 10.1016/j.coviro.2017.09.004
[27] Hui Ju Han, Hong Lin Wen, Chuan Min Zhou, Fang-Fang Chen, Li-Mei Luo, Jian-wei Liu, and Xue-Jie Yu, “Bats as reservoirs of severe emerging infectious diseases, Virus Res. 205, 2015: 1-6. doi:10.1016/j.virusres.2015.05.006
[28] See Endnote 17
[29] See Endnote 17
[30] Gerardo Martin, Carlos Yanez-Arenas, Carla Chen, raina K. Plowright, Rebecca J. Webb, and Lee F. Skerratt, “Climate Change Could Increase the Geographic Extent of Hendra Virus Spillover Risk,” EcoHealth 15, 2018: 509-25. doi: 10.1007/s10393-018-1322-9
[31] Ibid
[32] David W. Redding, Peter M. Atkinson, Andrew A. Cunningham, Gianni Lo Iacono, Lina M. Moses, James L. N. Wood and Kate E. Jones, “Impacts of environmental and socio-economic factors on emergence and epidemic potential of Ebola in Africa”, Nat Commun. 10, 2019: 4531.
[33] Ibid
[34] Jing Liu-Helmersson, Mikkel Quam, Annelies Wilder-Smith, Hans Stenlund, Kristie Ebi, Eduardo Massad, Joacim Rocklöv, “Climate Change and Aedes Vectors: 21st Century Projections for Dengue Transmission in Europe,” EBioMedicine 7, 2016: 267-77.
[35] Ibid
[36] Erin A. Mordecai, Jeremy M. Cohen, Michelle V. Evans, Catherine A. Lippi, Kerri Miazgowicz, Courtney C. Murdock, Jason R. Rohr, Sadie J. Ryan, Van Savage, Marta S. Shocket, Anna Stewart Ibarra, Matthew B. Thomas, and Daniel P. Weikel, “Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models”, PLoS Negl Trop Dis. 11, 2017: e0005568.
[37] World Economic Forum, Accessed on July 6, 2020.
[38] Ibid
[39] Sadie J. Ryan, Colin J. Carlson, Erin A. Mordecai, and Leah R Johnson, “Global expansion and redistribution of Aedes-borne virus transmission risk with climate change”, PLoS Negl Trop Dis. 13, 2019: e0007213.
[40] Ibid
[41] The Lancet Countdown 2019 Report. Accessed on July 6, 2020.
[42] Ibid
[43] Intergovernmental Panel on Climate Change 2019 Report. Accessed on July 6, 2020.
[44] Zhi-Ping Zhong, Natalie E. Solonenko, Yueh-Fen Li, Maria C. Gazitúa, Simon Roux, Mary E. Davis, James L. Van Etten, Ellen Mosley-Thompson, Virginia I. Rich, Matthew B. Sullivan, and Lonnie G. Thompson, “Glacier ice archives fifteen-thousand-year-old viruses,” bioRxiv 2020.01.03.894675.
[45] Matthieu Legendre, Julia Bartoli, Lyubov Shmakova, Sandra Jeudy, Karine Labadie, Annie Adrait, Magali Lescot, Olivier Poirot, Lionel Bertaux, Christophe Bruley, Yohann Couté, Elizaveta Rivkina, Chantal Abergel, and Jean-Michel Claverie, “Thirty-thousand-year-old distant relative of giant icosahedral DNA viruses with a pandoravirus morphology”, Proc Natl Acad Sci (USA) 11, 2014: 4274-79.
[46] Matthieu Legendre, Audrey Lartigue, Lionel Bertaux, Sandra Jeudy, Julia Bartoli, Magali Lescot, Jean-Marie Alempic, Claire Ramus, Christophe Bruley, Karine Labadie, Lyubov Shmakova, Elizaveta Rivkina, Yohann Couté, Chantal Abergel, and Jean-Michel Claverie, “In-depth study of Mollivirus sibericum, a new 30,000-y-old giant virus infecting Acanthamoeba,” Proc Natl Acad Sci (USA) 112, 2015: E5327-35.
[47] Melody Schreiber, “The next pandemic could be hiding in the Arctic permafrost”. Accessed on July 6, 2020.
The views expressed above belong to the author(s). ORF research and analyses now available on Telegram! Click here to access our curated content — blogs, longforms and interviews.

 PDF Download
PDF Download





 PREV
PREV


