
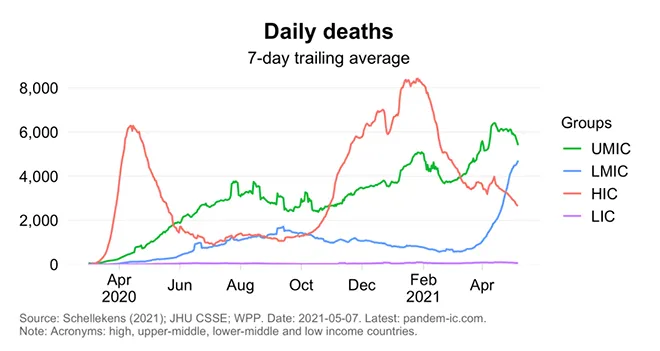
Earlier this year, stark inequities of global vaccine distribution, where limited supplies of COVID vaccine doses are mopped up by richer countries had forced the WHO chief to warn that the world was on the brink of a “catastrophic moral failure”. Lack of access to COVID-19 vaccines is now playing out across the world in terms of daily deaths spiralling out of control in relatively poorer countries, while vaccination efforts have resulted in significant reduction of deaths in the richer countries (Graph 1). The upper middle income countries have also seen daily deaths plateau and start declining, albeit with a time lag, in tandem with global vaccine distribution statistics. There is dawning realisation that without ramping up vaccine supply considerably and improving physical and financial access, the world cannot get out of the clutches of this pandemic. However, big pharma is largely looking the other way, blinded by huge profit margins.
 Source: https://pandem-ic.com/mortality-trackers/
Source: https://pandem-ic.com/mortality-trackers/
From the last quarter of 2020, it had become increasingly clear that the world’s ability to fight this pandemic will entirely depend on how the pharmaceutical sector and governments dramatically increase vaccine production capacity across the globe. The recent announcement by the US to partially support the joint proposal for patent waivers—introduced in October 2020 before the World Trade Organisation (WTO) by South Africa and India—has come as a surprise to many experts who have called it everything from ‘stunning’ to ‘symbolic’. The original proposal was to cover patents, industrial designs, copyrights, and protection of trade secrets across vaccines, medicines and diagnostics while the current US support is for waiving intellectual property rights in vaccines. Following the US turnaround after being largely absent from COVID-19 diplomacy, the Gates Foundation also made a somewhat surprising announcement that it supports a ‘narrow’ waiver on intellectual property protections during the pandemic.
It was announced by the WHO last month that of 700 million vaccine doses globally administered, only a shocking 0.2 percent had been in low-income countries. This is particularly concerning, as the pandemic itself is shifting from the richer world to the poorer world. There seems to be a consensus among experts that nobody is safe in the world till everybody is safe, since large pools of infection could continuously spawn new variants, pulling the world into a self-perpetuating spiral of mortality and uncertainty. Even without waiving intellectual property protections, Compulsory Licenses (CLs) are possible within countries, but a global waiver would facilitate exports as well, and incentivise scale-up without fear of legal implications. The dance of death that we see now in Indian cities, for example, could, perhaps, have been largely avoided if India’s October 2020 joint proposal at the WTO was accepted resulting in expanded production of vaccines.
India’s Efforts to Navigate the Global Pharma Ecosystem
Stalling any progress that threatens profit even remotely is a popular long-range weapon in the quiver of big pharma. From the beginning of the pandemic, they have been raising the issues of complexity of manufacturing processes and the perceived lack of capacity in the developing world. In a situation where expanding production quantities is a non-negotiable with poorer countries struggling to access doses, invoking quaint quality or capacity concerns in a way to prevent any such move is a disingenuous way of using the perfect as the enemy of the good. Even as its role as a major supplier of vaccines to the developing countries was disrupted by a disastrous second wave, India seems to be making clearly calculated moves towards global vaccine equity without much fanfare, supplementing efforts like COVAX.
One problem with the nature of the COVID-19 vaccine shortage is that there is a huge chance that ideal victories on paper will not translate into solutions on the ground quick enough, when the world needs them the most. Time is of the essence; and hence rather than symbolic moral victory of a waiver of all intellectual property protections on COVID-19 solutions, partnerships that are built on a range of voluntary licenses, technology transfer, and consolidation and expansion of manufacturing capacity in the developing countries would be a more appropriate, and parallel goal. Apart from a handful, like the ones involving Serum Institute of India, such partnerships have not been forthcoming in the past.
The Primacy of Timely Tech Transfer
Technology transfer, a key component for timely manufacturing of vaccines, will help developing country manufacturers save months if not years of time. With a raging pandemic, a looming threat of waivers or CLs may be more useful than actual waivers and CLs per se. If some hard bargains involving technology transfer, voluntary licenses, and expansion of manufacturing capacity in the developing world can be arrived at using these levers, that should be the real negotiation the world needs. With major players like the US and Gates Foundation changing their position rather dramatically, there will be pressure—political and moral—on players like the European Union (EU), Britain and Japan to play ball.
As the only developing country member of The Quad, India has been playing an extremely important role behind the curtain in facilitating the partnership between Johnson & Johnson and Biological E, and securing US support to produce 1 billion doses to strengthen multilateral initiatives like COVAX.
If the changed regulatory environment and the pressure mounted by countries led by India and South Africa leads to a range of strategic voluntary licenses across the world, and results in strengthening of the COVID-19 Technology Access Pool (C-TAP)—a WHO initiative to facilitate technology transfer, unpopular till now with the industry—that will be a victory for global public health. Pressure is building on multiple fronts: With Indian company Natco filing for CL for the INR 42,000 per-patient treatment regimen COVID-19 drug Olumiant (Baricitinib) by Eli Lilly, and similar discussions across the world, it is expected that big Pharma will have to act fast to retain credibility and enhance access to vaccines and drugs.
Despite submitting the joint proposal at the WTO, which is gaining support from the unlikeliest of places due in part by its silent, backroom diplomacy, India also seems to have realised that the global pandemic is not to be seen solely as an opportunity to quickly reform IPR ecosystem, and has leveraged its position as a major global manufacturer well. India jointly owns the rights of the indigenous Covaxin vaccine with Bharat Biotech, and currently the production is being ramped up with three public sector companies namely Indian Immunologicals Ltd (IIL), Bharat Immunologicals & Biologicals Corporation (BIBCOL) and Haffkine Biopharmaceutical Corporation planning production of millions of doses every month, along with Bharat Biotech. Reports indicate that Bharat Biotech is also in talks will global companies to enhance production.
The First People’s Vaccine?
India is moving slowly but surely using a multi-pronged approach— first by creating pressure on the established players with a joint WTO proposal which has increasingly gained supporters, then by creating goodwill by exporting a large proportion of vaccines produced at immense domestic political costs, and at the same time, leveraging its strategic position by making possible the Quad supported J&J production in India, followed by playing a role in getting the US government, Gates Foundation and GAVI to support a subset of the original—radical—proposal. The short-and medium- term outcomes of such an approach may, perhaps, be less revolutionary, but these will be certainly more practical, and with a higher impact on the pandemic. Once the much awaited final results of Covaxin’s Phase 3 Trial are published, it is expected that in the true spirit of “Vasudhaiva Kutumbakam” (the whole world is one single family) India may offer Covaxin to the world as the first truly global “people’s vaccine”, an act which will have great historical and symbolic value.
There is already consolidation of production capacity of vaccines happening globally as well as in India, with Merck choosing to produce Johnson & Johnson’s vaccine, multiple companies in India producing Covaxin, and other instances of licensed manufacturing. Scaling and broad-basing these efforts to enhance production capacity in the developing countries, supported by technology transfer is the challenge. Given the shift in the position of key players and pressure on major pharmaceutical companies to show social responsibility, freeing up intellectual property rights of Covaxin, can possibly be a catalyst for bigger things with the support of platforms like C-TAP.
Earlier this month, Moderna published its revised 2021vaccine sales forecast at US $19.2 billion, after Pfizer shared its sales forecast at US $26 billion. Greed could become a major roadblock to global vaccine equity, but a realignment of forces may help the kind of solutions India is trying to forge, leveraging its geo-strategic positioning as well as existing production capacity which is being further enhanced with support from strategic partners like Japan on the one hand, and threatening to upset the applecart of the big pharma through disruption, on the other. A rare historical event when self-interest and equity align, this pandemic requires active sharing of knowledge and resources across humanity, not half-hearted statements from big pharma that patents won’t be enforced on ‘rivals’.
If everything goes well, a rather narrow WTO waiver limited to vaccines may come about in time after longish negotiations, which will act as a bonus providing “a measure of certainty to manufacturers and governments to start investing in new facilities and develop cold storage chains”, according to analysts. Till then, many more voluntary licenses will have been granted, supported by active technology transfer as well as capacity expansion, already multiplying the global output of COVID-19 vaccines many times over. Indeed, the perfect need not be the enemy of good, and despite being debilitated by a destructive surge of the pandemic, India has been cautiously moving towards global vaccine equity against almost impossible odds, achieving one small victory at a time. When the world’s access to health is mediated by big pharma interests, the developing world badly needs not just a fair share of the vaccines, but also a fair share of the right to produce the vaccines.
The views expressed above belong to the author(s). ORF research and analyses now available on Telegram! Click here to access our curated content — blogs, longforms and interviews.




 Source:
Source:  PREV
PREV


