-
CENTRES
Progammes & Centres
Location
Yesterday, the University of Oxford, in collaboration with the British-Swedish pharmaceutical firm AstraZeneca, announced the interim results from the Phase 3 trials of their COVID-19 vaccine conducted across the UK, Brazil and South Africa. Analysis of 131 COVID-19 cases from both arms of the trial indicates that the vaccine is overall 70.4 percent effective. According to a press release, further trials are being conducted in the United States, Kenya, Japan and India and is expected to have under 60,000 participants by the end of the year. These efficacy numbers are based on preliminary results; and final trial results are awaited.
Results from the Oxford-AstraZeneca trials closely follow very promising early data on the efficacy of three other major vaccines from Pfizer, Moderna and Gamaleya of Russia. All three have claimed that their vaccines have more than 90 percent efficacy in preventing COVID-19. Oxford-AstraZeneca have announced that despite an overall efficacy of 70.4 percent, their vaccine can offer “upto 90 percent protection” by administering a half dose followed by a full dose instead of two full doses. However, there is skepticism around this claim, primarily since this was discovered as a result of a dosing error in the trial.
Considering that the World Health Organisation (WHO) had earlier suggested an efficacy cut-off of 50 percent as the minimum criterion for any acceptable COVID-19 vaccine, these preliminary results look extremely promising across the board. However, the answer to the question of when exactly people will get the vaccine will mostly depend on where they live and if they are identified as highly vulnerable to the coronavirus; this will be the case for at least a year, if not longer.
As of now, there are 13 vaccines across the world in Phase 3 clinical trials, and the preliminary results of the efficacy of a handful have been shared with the public. Of these, Moderna’s vaccine development is supported by the United States government, which has already committed billions of dollars to the effort. Last week, Moderna announced that their vaccine was 94.5 percent effective. Pfizer and the German company BioNTech are collaboratively developing the second vaccine candidate whose interim efficacy numbers are also out. Pfizer and BioNTech have announced that their vaccine is 95 percent effective and that even among the elderly, who normally have weak responses to vaccines, it is almost as good.
The Gamaleya Research Institute, part of Russia’s public sector, has published efficacy numbers from the Phase 3 trial of their Sputnik V vaccine, and based on 20 cases of COVID-19 among the 16,000 trial participants, its efficacy stands at 92 percent.
The Gamaleya Research Institute, part of Russia’s public sector, has published efficacy numbers from the Phase 3 trial of their Sputnik V vaccine, and based on 20 cases of COVID-19 among the 16,000 trial participants, its efficacy stands at 92 percent. AstraZeneca and the University of Oxford are the latest to share interim results from their Phase 3 trials. According to them, the vaccine has an overall efficacy of 70 percent. At the same time, different dosing regimens have been shown to produce different efficacy results ranging from 62 percent to 90 percent, a fact that has puzzled experts across the globe. Reportedly, the Oxford-AstraZenenca vaccine offers protection for the elderly population just like the Pfizer vaccine, something that we don’t know yet about the Moderna and the Gamaleya vaccines.
Rapid vaccine development for COVID-19 has been the result of unprecedented global cooperation amongst the international scientific community. However, as more and more vaccines are establishing efficacy, a growing number of countries are opting for a “my nation first” approach. As rich countries chase vaccines, apprehensions have been raised from multiple quarters, including the WHO, that the risk of the poorest and the most vulnerable people ‘being trampled in the stampede for vaccines’ is very real. This is despite the existence of the Access to COVID-19 Tools (ACT) Accelerator facility jointly led by the WHO and a vaccine arm (COVAX) that coordinates the collective procurement and distribution of vaccines for the most vulnerable populations within poorer countries across the globe, whose aim it is to distribute 2 billion doses by the end of 2021.
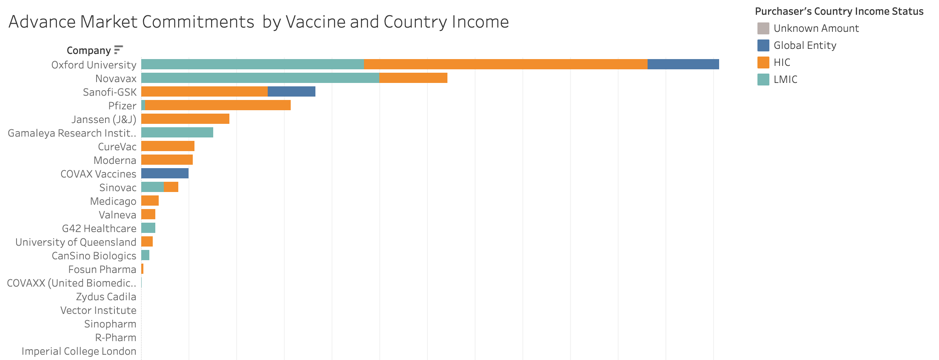
However, most rich countries and those with production capacity have already made elaborate arrangements in terms of advance market commitments (AMCs) with vaccine manufacturers. In total, 9.8 billion vaccine doses of those candidates in Phase 3 trials have already been reserved, according to publicly available data. This is reminiscent of the situation earlier on in the pandemic, when the United States bought up around 90 percent of the world's supply of Remdesivir, which left very little for the rest of the world for months. Pfizer and Moderna has AMCs almost exclusively with high income countries (Graph 1), given the high price of their vaccines, and possibly also because of the challenging cold-chain requirements.
Graph 1: Covid19 Vaccine Advance Market Commitments Across Companies
 Source: Duke Global Health Innovation Centre
Source: Duke Global Health Innovation Centre
Publicly available data suggests that high-income countries have secured a confirmed 3.7 billion doses of the vaccines under advanced phases of clinical trial, upper middle-income countries have secured 706 million doses, and lower middle-income countries have secured more than 1.7 billion doses—in bilateral arrangements with vaccine producers. It is likely that low-income countries will have to rely predominantly on the COVAX facility for their coverage for the time being. Vaccines produced by India and Russia could hopefully ensure global access. China is also expected to roll out vaccines to developing countries through its health diplomacy efforts.
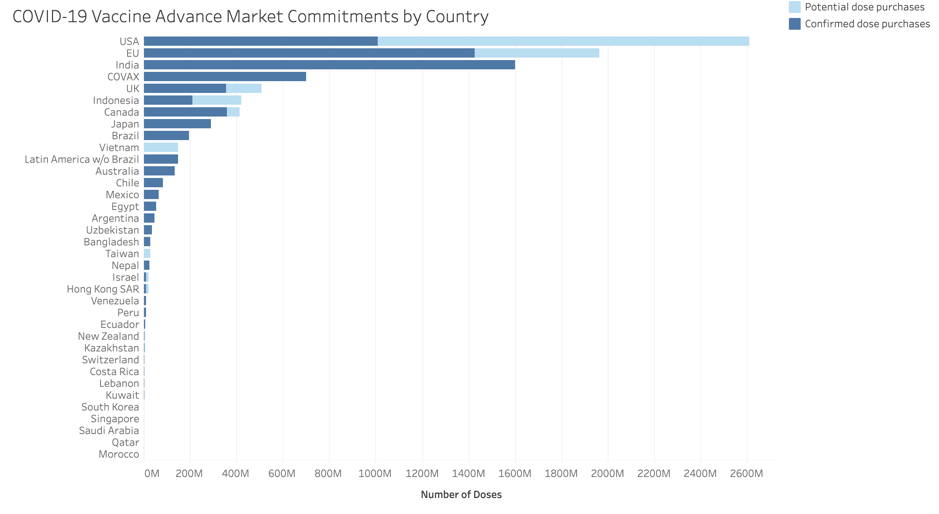
In total, India has 1.6 billion confirmed doses of COVID-19 vaccines currently in Phase 3 trials, while the European Union and the USA follow closely behind with 1.4 billion doses and 1.01 billion doses respectively
In a situation of too many dollars chasing too few vaccines in Phase 3 trials, available data reveals that India has surprisingly emerged with the highest number of confirmed dose purchases (Graph 2) in the world, leveraging its own production capacity. India has already secured half a billion doses of the Oxford-AstraZeneca vaccine, one billion doses of the Novavax vaccine, and 100 million doses of the Sputnik-V vaccine. In total, India has 1.6 billion confirmed doses of COVID-19 vaccines currently in Phase 3 trials, while the European Union and the USA follow closely behind with 1.4 billion doses and 1.01 billion doses respectively. Along with the extremely high costs of some of the lead vaccines, logistical challenges of ensuring ultracold cold chain facilities seem to have influenced India’s options.
Graph 2: Covid19 Vaccine Advance Market Commitments Across Countries
 Source: Duke Global Health Innovation Centre
Source: Duke Global Health Innovation Centre
According to reports, the Pfizer and Moderna vaccines will cost around $19.50 and $25-$37 per dose respectively. When cold chain requirements are added, these become prohibitively expensive for low and lower middle income countries. While its cold chain requirements are similar to that of the Moderna vaccine, Sputnik is to be priced much lower, according to Russian officials. It is here that the Oxford-AstraZeneca vaccine has a distinct advantage as along with its low cost, the vaccine will not need sub-zero storage facilities.
A major risk is that of people hearing about high efficacy vaccines day in and day out on the media and then deciding that the pandemic is all but over. The development of a vaccine is one thing, but getting huge populations across the world vaccinated could take months if not years. With the newfound confidence that vaccines give, it will be a big challenge to get across risk communications for people to stick to COVID-19 protocols and stay indoors. We will need calibrated communication strategies aimed at ensuring that the cheer for new vaccines does not get converted into spikes in COVID-19 cases in the coming months.
Taking leadership, India and South Africa requested the World Trade Organisation (WTO) to allow countries to opt for non-enforcement of intellectual property rights for COVID-19 related innovations including vaccines for the duration of the pandemic, until the world achieves herd immunity.
Despite many successful vaccines, enforcement of strict intellectual property rights could mean that timely access to the vaccine(s) by a majority of the global population may not be possible. Taking leadership, India and South Africa requested the World Trade Organisation (WTO) to allow countries to opt for non-enforcement of intellectual property rights for COVID-19 related innovations including vaccines for the duration of the pandemic, until the world achieves herd immunity. As argued forcefully elsewhere, to achieve the objective of aligning global and national goals, countries at the forefront of the fight against the pandemic, like India, will have to pool the strengths of its public and private sectors into a National Anti COVID-19 Mission platform.
COVID-19 vaccines are a global public good that will directly contribute to the economic revival of the world. It is time for countries, including India, to consider people across their border as their own citizens. Perhaps, it will mean a vaccine delivery protocol that prioritises high risk populations across the border ahead of low-risk populations within the country. In this vein, Prime Minister Narendra Modi has been the first world leader to unequivocally announce that the country’s vaccine producing capacity will be used to help the entire humanity in fighting COVID-19. It needs to be ensured that the unprecedented process of the ‘vaccination of the world’ happens with a sense of collaboration and cooperation, protecting ethics rather than profits, reflecting PM Modi’s words. As said many times over during this pandemic by many, no one is safe until everyone is safe.
The views expressed above belong to the author(s). ORF research and analyses now available on Telegram! Click here to access our curated content — blogs, longforms and interviews.

Oommen C. Kurian is Senior Fellow and Head of Health Initiative at ORF. He studies Indias health sector reforms within the broad context of the ...
Read More +