
Children as young as three and six are rolling up their sleeves to receive jabs of COVID-19 vaccine candidates as the worldwide treasure hunt for a cure expands to younger people, and concerns spiral about turning the corner at population scale.
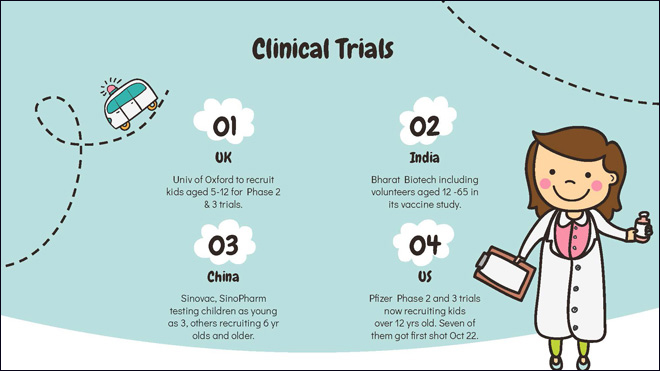
The first wave of vaccines will be for adults and cannot be given to kids, unless they've been tested in that age group. That process has now kicked off. In the US, Pfizer has begun recruiting volunteers 12 years and older for its phase two and three trials. Moderna, Johnson and Johnson and Novavax are all hoping to begin pediatric studies later in the year. With cooler weather in the Fall, doctors are stepping up warnings of a “dark winter” ahead as schools are reopening for hybrid learning.
The Cincinnati Children's Hospital has expanded its Pfizer clinical trial to include 12-15-year-olds after first enrolling 16-17-year-olds. Seven children between the ages of 12 and 15 got their first shots on October 22.
Doctors leading the Pfizer trial at Cincinnati Children's Hospital are hoping for a best case scenario, where the vaccine activates up adolescents’ immune systems the same way it has been doing in adults, without wild side effects. They are hoping to have answers for the 12 and older group by Spring 2021.
University of Oxford researchers will recruit children aged 5-12 into a phase 2/3 trial of its vaccine. China and India studies are also including children in COVID-19 vaccine trials, some as young as six.
However, scientists still don’t know how well a vaccine would protect from the disease itself and from infection.
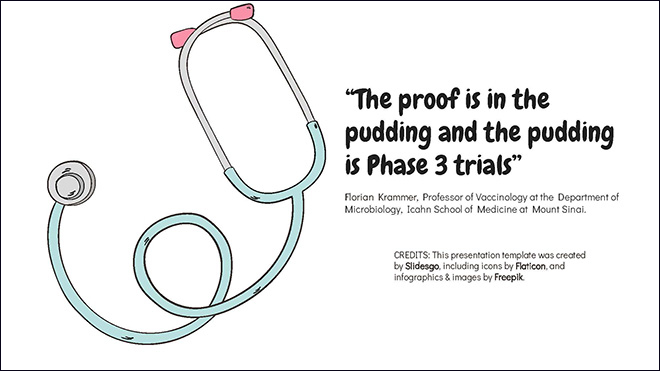
“The proof is in the pudding and the pudding is Phase 3 trials” is how Professor Florian Krammer described the grand challenge, during an overview of ongoing trials. Krammer is Professor of Vaccinology at the Department of Microbiology, Icahn School of Medicine at Mount Sinai.
Krammer made a note about the inflammatory response to vaccination being on the higher side, and how that’s not a problem for adults but raises questions around usage and acceptability for kids.
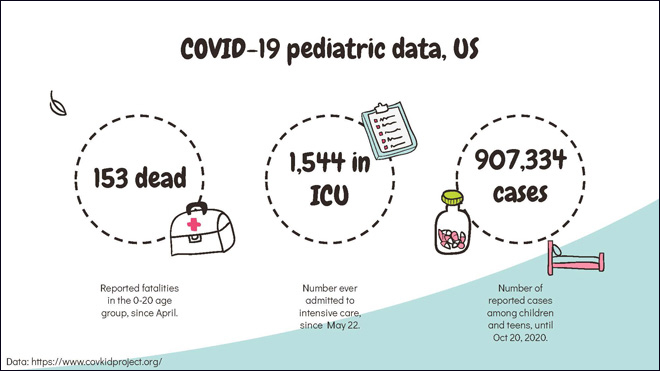
Children represent about 10% of documented COVID-19 cases in the US, according to an AP report. The COVKID project reports that 153 children in the 0-17 age group have died since April. Since May 22, 1,544 children have been admitted to intensive care. In the six and a half months from March to mid September 2020, 277,285 COVID-19 cases have been reported in the US, according to the Centers For Disease Control and Prevention. Within that dataset, the attack rate in the 12 to 17 age group was double that of children in the five to 11 years age group.
In the US, at least 5 vaccine candidates are in Phase 3 trials. The vaccine made by Pfizer and its German partner BioNTech are among several leading candidates in final testing. Two studies which started trials on July 27 are fully enrolled and ready to start collecting data.
Dr. Anthony Fauci, the top infectious diseases expert on the White House coronavirus task force, said we’ll likely know about safety latest by early December and that vaccine doses will be made and ready to go by the end of the year for frontline workers and the most vulnerable.
Some of the standout data on COVID19 in kids and the vaccine in the works are in the charts below.

The views expressed above belong to the author(s). ORF research and analyses now available on Telegram! Click here to access our curated content — blogs, longforms and interviews.








 PREV
PREV


