Introduction
The turbulent waters of the Bay of Bengal (BoB) regularly witness natural calamities, particularly cyclones. It is thus a permanent sink for public funding for the BoB littorals to mitigate crisis situations that require humanitarian response. At the same time, however, the sea holds promise for the prosperity and growth of the region, particularly through the development of marine fisheries and other allied industries. The shallow coastal waters on the northern edge of the BoB, shared by West Bengal and Bangladesh, a rich variety of aquatic life,[a] much of which utilises the sanctuary provided by the Sundarbans, the world’s largest tidal delta, as a nursery for breeding. The species or groups that are commercially important include the Hilsa Shad, Bombay Duck, Cat Fishes, Silver Pomfret, Indian Mackerel, and Penaeid Prawns. Despite having the shortest of coastline amongst all coastal states in India and a paucity of land due to high density of population, West Bengal, in 2017–18, was the second-highest fish-producing state in the country with an output of 1.7 million tonnes;[1] Bangladesh produced about 4.2 million tonnes.[2]
On the other hand, its share of marine fisheries has been abysmally low. For the same years, the production of marine fisheries in West Bengal and Bangladesh have been about 0.19 million tonnes and 0.65 million tonnes, respectively, representing 11 percent and 15 percent of the total share. Much of this resource extraction is concentrated inshore, about 30 and 60 nautical miles from the shore, respectively, and in the much shallower estuarine waters. The activity is thus highly concentrated and target-specific, often focused on the commercially important species such as the hilsa shad. Consequently, the transboundary fish have been overharvested[b] in the northern part of the Bay of Bengal in both India[3],[4] and Bangladesh.[5],[6],[7]
The Problem
The common response to this problem is to rely on the principles of conventional management of fisheries, which dictates where, when, and how much to fish of one particular kind. Management options have been overly reliant on regulatory instruments: fishing bans for specific periods; monitoring of fishing practices, such as prohibiting the use of certain fishing nets for reducing bycatch and catching the juvenile fishes; and strict patrolling of the seas to prevent foreign fishers from fishing in territorial waters.
Bangladesh passed the Protection and Conservation of Fish Act, 1950 which bans the catching, transportation, marketing, selling, and processing of juvenile hilsa or jatka between 1 November and 31 May each year.[8] Since 2001, the Bangladesh Navy, along with other law enforcement agencies including the Police, Coast Guard, and Naval Police Border Guard, have been conducting two annual operations to implement the rule of law—Operation Jatka and Operation Maa Elish Rokksha. In 2020, the Bangladesh Air Force also participated in the operation through aerial monitoring. Similarly, the West Bengal government has imposed a ban on fishing during June–August and October–December, to facilitate spawning in the hilsa sanctuaries, and issues directives for regulating the mesh size to protect juveniles.[9]
Another specific intervention was the naming of six sections of the rivers Padma, Meghna, Tetulia and Andharmanik as “hilsa sanctuaries.” In 2013, West Bengal issued a circular declaring stretches of the Bhagirathi-Hooghly river as hilsa sanctuaries.[10] In Bangladesh, a prolonged ban is imposed in hilsa sanctuaries from November to June, and adaptive co-management of fisheries is practised with communities engaged in the activity.[11] Additionally, there is a two-month-long fishing ban (March and April) in all sanctuaries, except the Ardhaemanik River. Another 22-day nationwide ban on the catch, transportation, and sale of hilsa is enforced during the full moon period of October to protect the breeding of hilsa in Bangladeshi waters.
While species-specific interventions that are targeted at reducing overexploitation is a necessary condition for the sustainable management of fisheries, it is not sufficient. This is because of the threats currently faced by the target species, for example, hilsa, and its habitat, in the backdrop of larger anthropogenic interventions in the natural systems, both local and global.
Similarly, for gauging the health of fish stocks, a common practice is to calculate the Catch Per Unit Effort (CPUE), a ratio used to eliminate temporal trends in simple fish stock abundance. The “catch” segment is the number or weight of the entire catch, or a selected subset or specific species in the catch. The “unit effort” segment refers to a uniform piece of gear employed to catch fish such as the number of vessels, vessel days, trawl, and gillnets. While the CPUE is a powerful tool, and a decline in CPUE is usually a good indication that stocks are declining, the assumption that the abundance of fish stock has a direct relationship with CPUE may not be accurate.[12] Situations involving highly gregarious and schooling species, one of which is also the hilsa shad, may indicate a high CPUE (or stock abundance) even when the resource has already been overexploited. To overcome this challenge, it is important to have participatory engagement with fisherfolk to perform controlled experiments and fishing in separate areas and using new ways.[13] Moreover, disaggregated data for calculating both catch and effort point to a need for a robust process of standardisation. Using a simple process to aggregate and compute CPUE can give inaccurate results.
Thus, fisheries management requires an urgent shift in paradigm and the adoption of a more holistic approach. The capture of marine fish and other species are the provisioning services of a healthy, self-sustaining marine ecosystem. (See Figure 1). Changing the way the resource is perceived (as a product of ecosystem functions and processes) and attuning it with the scientific realities of anthropogenic influence adversely impacting the ecosystem will make it evident that the resource is essentially finite. The bottom line is that there exists a threshold beyond which harvestation or external changes in the ecosystem will affect ecosystem function and productivity, and make irreversible changes to it, thereby affecting the availability of fish.
Figure 1: Interdependence between Human Systems and Marine Systems[14]
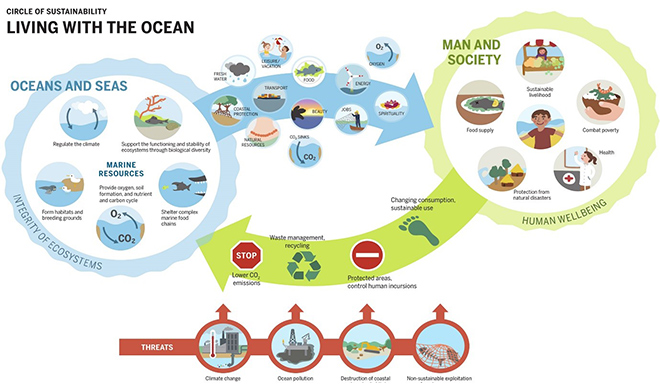
Source: Ocean Atlas (2017)
This paper argues that the conventional approach is inadequate to deal with the multitude of threats affecting ecosystem processes and functions, highlighting the local and global anthropogenic interventions to natural systems and processes. The paper builds a case for an “Ecosystems Approach to Fisheries Management” (EAFM) by taking the case of the anadromous hilsa shad (Tenualosa ilisha). This species was chosen because there is enough data. Owing to its high commercial value, the hilsa has been the subject of extensive research, and there is a repository of data on the status of the fish and the issues facing the industry. The paper further argues that increased participation of local stakeholders, especially the fisherfolk, in drafting the rules for management would allow for better compliance of such rules, compared to the current top-down paradigm.
Hilsa Shad: Mainstay of a Highly Lucrative Industry
The Hilsa shad or the Tenualosa ilisha is an extremely sought-after fish in West Bengal and Bangladesh for its high commercial value. A favourite and an integral component of Bengali cuisine, especially during the monsoon season, the fish has also been at the centre of Bangladesh’s “Hilsa Diplomacy.” Bangladesh has repeatedly lifted its ban on Hilsa exports to India, to foster goodwill—especially with West Bengal, with which it shares transboundary rivers including the Teesta,[15] and also other states like Tripura and Assam.
Contrary to popular notions, the fish species is not endemic to the Bengal coast and is widely distributed along the coasts of the Persian Gulf, the Arabian Sea, and the Bay of Bengal. Being anadromous, a large share of the fish population moves upstream along river channels to spawn in freshwater habitats and returns to its marine habitat with the juveniles. However, significant divergence from this trend has also been observed, highlighting that a subset of the population completes its entire life cycle within freshwater habitat while another subset completes it within the marine environment and moves to the estuarine waters for spawning.
The habitat of the Hilsa includes its entire geographic reach, until about 410 river kilometre (rkm) upstream in Hooghly, 420 rkm in the Padma, 780 rkm upstream in Brahmaputra till Tezpur, 275 river kilometre upstream in Meghna, and 825 rkm in Irrawaddy till Mandalay.[16] In the marine waters, the seaward extent of the fish, particularly in its adult stage, extends to the shallow waters of the continental shelf.[17] There is also sufficient evidence to conclude that the schools of fish migrate between the spawning grounds in the Ganga delta and in the Irrawaddy delta,[18] signifying the shared nature of the resource between three countries—India, Bangladesh and Myanmar. An understanding of the geographic reach of the species is critical for identifying the threats to the marine ecosystem, in general, and the hilsa shad in particular.
Hilsa is extremely important for the economy of Bangladesh, and it constitutes the largest single-species fishery in the country. In 2017-18, its share was 12.09 percent of the total catch in metric tonnes, and Hilsa constituted more than half of all the marine fish production. This fish alone contributes more than one percent of the country’s GDP, provides employment to 0.46 million people,[19] and contributes to foreign exchange earnings of US$12.5 million per year. Indeed, the species has been conferred the title of “the national fish of Bangladesh,” and the country has been taking strong measures to conserve it within its waters.
The hilsa shad also features prominently in the basket of fish catches in West Bengal, at about 12.5 percent of the total catch.[20] However, the state currently imports a sizeable share from neighbouring Bangladesh and Myanmar. According to a 2019 report by the World Fish Centre, West Bengal’s consumption of the fish was at 100 tonnes per day between July 2012 and September 2012. Seven percent of this demand was fulfilled from Bangladesh alone, while the rest arrived from other landing sites in India. However, the authors have provided the disclaimer that this assessment considered only official channels, and the figure could be underestimated since the unofficial route is “cheaper” and “less complicated.”[21] Even in terms of consumer preference, the Bangladeshi hilsa is considered to be a delicacy and fetches a far higher price per kg (INR 2,500-3,000) than do the locally procured ones from the Hooghly-Bhagirathi (INR 1,500) and the coastal waters of West Bengal (INR 1,800).
The gap in demand and supply can also be understood from the absolute catch of the fish by the three main hilsa producing countries (See Figure 2). According to 2008 estimates, 50-60 percent of the global hilsa catch is from Bangladesh (3.5 lakh metric tonne/year), 20-25 percent from Myanmar (1.0-1.25 lakh metric tonne/year), 15-20 percent from India (0.50-0.60 lakh metric tonne/year) and 5-10 per cent from other countries (e.g., Iraq, Kuwait, Malaysia, Thailand and Pakistan).[22] While Myanmar has been a late entrant in this lucrative industry, it has managed to overtake India in a short period of time.
Figure 2: Year-Wise Total Catch (MT) of Hilsa from the BoB (1984–2013)[23]
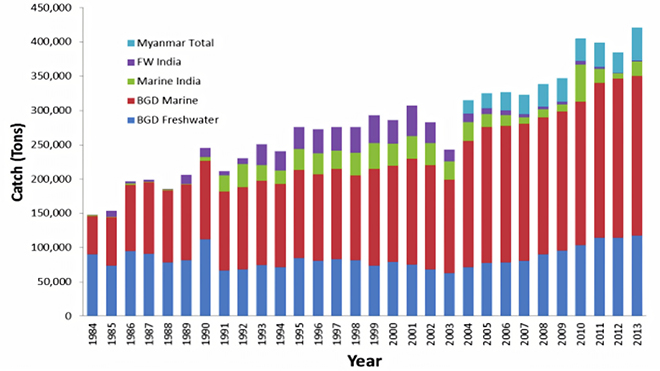
Source: Bay of Bengal Large Marine Ecosystem (2014)
Why Hilsa Catch is Dwindling
The production of hilsa in Bangladesh has steadily increased across marine and freshwater sectors. In sharp contrast to this, the production of hilsa in both India and West Bengal has registered a declining trend (See Figures 2 and 3) since 2002. Moreover, in the initial decade after the modernisation of the industry in the early 1990s, the freshwater catch of hilsa in India was comparable to the marine catch; however, over the years, there has been a marked decrease in the share of freshwater catch. Specifically in West Bengal, the overall catch dropped (See Figure 3) after a peak in 2002, mirroring India’s overall trend, and since 2009, the freshwater or inland catch from the Bhagirathi-Hooghly system constitutes a very small part of the total catch of hilsa in West Bengal. Indeed, this crisis of freshwater catch in the state is so acute that even in “good years” of hilsa production (for example, 2010), the overall increase was driven by a disproportionate increase in the marine catch and not riverine catch (See Figure 3).
Figure 3: Year-wise Production (in Metric Tonnes) of Hilsa in the Hooghly-Bhigirathi River System (Inland) and Nearshore Areas (Marine)[24]
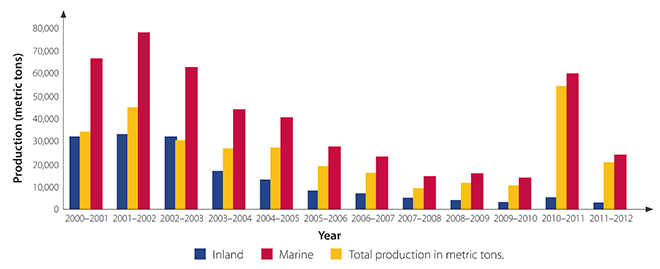
Source: WorldFish
Another way to understand the declining catch in the inland waters is by examining the CPUE data for various landing sites. Sajina et al. (2019) estimated the CPUE for gillnet at important landing stations during the two peak fishing months—monsoon and winter—and over a period of four years (2013–16). In a general finding, it was observed that for both the seasons, the CPUE showed a gradually declining trend as one moved upstream of the river. Digha and Fraserganj, located on the coast and in the estuary respectively, recorded the highest values; Balagarh and Farakka, located far upstream, recorded the lowest value (See Figure 4).
Figure 4: Migration Route of the Hilsa Shad in Bhagirati-Hooghli[25]
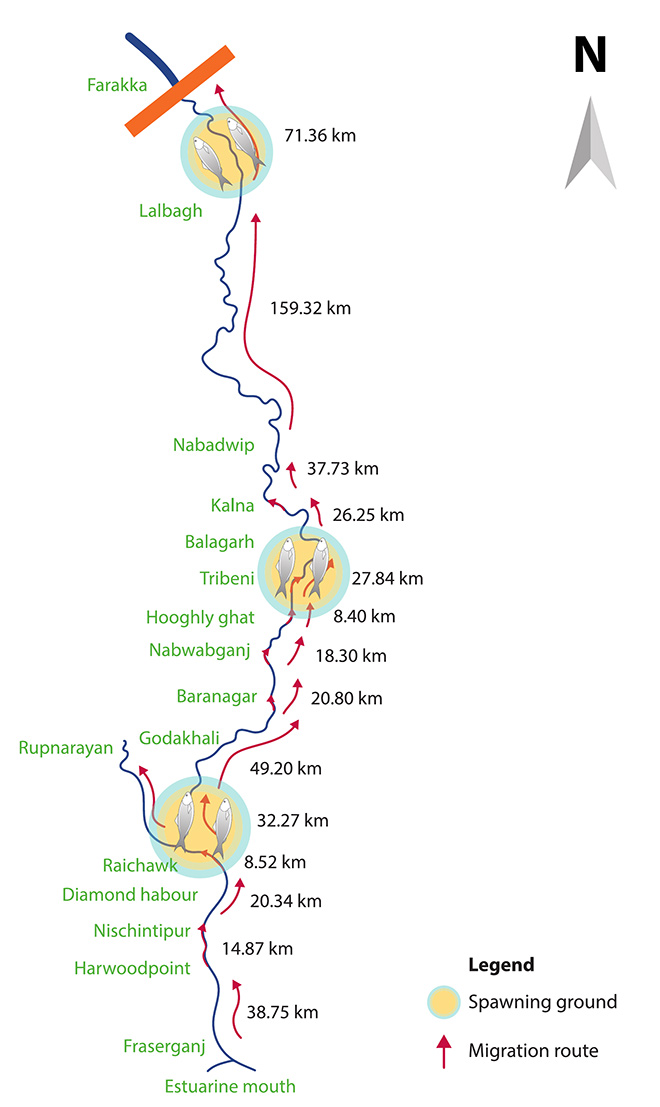
Source: WorldFish
An explanation for this apparent discordance in the inland catch of hilsa between adjoining West Bengal and Bangladesh is the forward-looking vision and concerted action of the Bangladeshi government towards conserving the fish and protecting the juveniles. For example, the government-sponsored “Hilsa Fishery Management Action Plan (HFMAP),” implemented in 2005, was instrumental in revitalising the stock in Bangladeshi waters. An important feature of the plan has been the “stick and carrot” approach, i.e. not only implementing a ban on fishing but also providing incentives, which includes 40kg of rice/month for seven months covering the entire ban period and enabling Alternative Income Generation Activities (AIGA). The HFMAP was followed by the Enhanced Coastal Fisheries in Bangladesh (ECOFISH-Bangladesh), which was operational between 2014 and 2019.[26] Compared to these initiatives in Bangladesh, the conservation efforts in West Bengal have remained patchy and law enforcement is poor.[27]
However, it can be argued that if the stock of the resource is essentially shared between India, Bangladesh, and Myanmar, then the depleted stocks in the Bhagirathi-Hooghly would be duly replenished from elsewhere.[28] However, this has not been the case as evident from data, despite the relatively stable catch in neighbouring Bangladesh. This points towards a crisis far greater than what species-specific interventions to replenish and revitalise Hilsa stocks could achieve. Evidence, essentially scattered, suggest that humans have altered the river channel to such an extent that the cumulative impact impedes the upstream migration of the hilsa.
- Elevation of riverbed and siltation in the mouth of the estuary of Hooghli-Bhagirathi. During the non-monsoon months, the depth at the mouth of the estuary drops to less than 10.0 feet (3.04 m), and even further to only knee-deep levels in certain places.[29]
- Construction of dams and barrages in the upper stretch of rivers, reducing the water flow requirement for spawning and migration of the hilsa. The Farakka barrage has impacted the ability of the fish to move upstream, and the use of remedial instruments such as fish locks has done little to alleviate the problem.[30]
- Loss of habitat due to increased water abstraction for human use such as irrigation. There is inadequate water during the non-monsoonal season and a decline in water depth in the river channel. The accumulated silt chokes the passage of the hilsa fish, which require a depth of 4-5 m for upstream migration and a water column of 18-20 m for stress-free movement of brood stocks. Consequently, the size of the migratory hilsa during winter is smaller than in the monsoon period.[31]
- The extent and periodicity of pollution in the river due to industrial and domestic effluents impedes the stress-free migration of the hilsa.[32] The physiochemical parameters of water (transparency, salinity, specific conductivity, alkalinity, hardness, dissolved oxygen, and water temperature—order of significance: high to low) play an important role in determining the abundance of fish or CPUE in tidal and non-tidal stretches of the river.[33]
The Freshwater-Marine Continuum
The management of fisheries should expand from its narrow and reductionist form in order to address the threats currently affecting the industry. This is evident from the hilsa case study and the learnings can be extrapolated to all commercially important aquatic species being exploited in the region – all of which are essentially transboundary resources, fluid in their movement within the shared coastal waters. To this end, a proper understanding of the ecosystems of which such species are part and the processes that sustain them becomes paramount. In this section and the subsequent one, freshwater, estuarine and marine environments have been identified along with the detailed explanations of the processes that are crucial for the functioning and productivity of the ecosystems these environments support. For instance, the hilsa can migrate long distances upstream, and unless impeded, the habitat could extend beyond the region mentioned here. However the delineation of the three distinct environments and continuum in this paper is required for the purpose of formulating a heuristic response in dealing with complex challenges of the region.
Based on its distinct submarine topography, hydrography, productivity, and dependence of population in the tropics, the Bay of Bengal Large Marine Ecosystem (BOBLME) has been identified as one of the world’s 64 globally significan Large Marine Ecosystems.[34] The Ganges-Brahmaputra Estuarine Front (GBEF), a part of the BOBLME, is a relatively shallow embayment that forms as an interface between the freshwater river outflow (plume) and the ambient sea water. It holds a particularly prominent position as it includes the outlet of the Ganga Brahmaputra Meghna (GBM) river system that brings not only freshwater but also sediments. Therefore, these are also turbidity fronts and can be easily observed from outer space due to their distinct colour and transparency gradients. The location of the GBEF coincides with the shelf break[35] and traces loosely the seaward boundary of an underwater delta of the GBM. It marks the southward extent of the space, since, beyond the GBEF, the salinity gradient increases manifold along with the depth. These characteristics restrict the movement of organisms adapted to the conditions of the shallow seas—a region that has large and permanent influence of freshwater, high sediment influx and high primary productivity. In terms of the net outfall, the GBM system ranks fourth in the world, with an annual average discharge of 1032 km3.[36] In terms of geological age, the formation is quite recent; starting in the Holocene epoch (over the last 11,700 years), the delta continues to prograde as a clinoform[c] and a sizeable part of it is submarine.
The progradation[d] extends the delta 125 km across the continental shelf from the mouth of the rivers and about 380 km along the shelf. It is bound by the Hooghly river in the west and the Tripura fold belt in the east (See Figure 5). The delta is incised by the submarine canyon or the “Swatch of No Ground” located within 30 km from the shoreline that acts as a conveyor to carry the sediments from the shallow part of the sea to the deeper waters .[37] The total area is about 140,000 km2, of which 110,000 km2 is subaerial,[e] while the rest of it is subaqueous or underwater. Moreover, much of the delta is within the border of Bangladesh while only 25,000 km2 or 22 percent of the subaerial delta is within West Bengal. The subaerial delta can be further conceived as constituting two distinct units—the Upper Delta Plain (UDP) and the Lower Delta Plain (LDP). The boundary between the two is defined by the beginning of offshoots from the Ganga to the BoB, and by a point of frequent channel avulsion[f] along the Brahmaputra.[38] The UDP is dominated by fluvial processes and are essentially freshwater stretches of rivers while the LDP is influenced by saline water from the sea. Until recently, much of the LDP consisted of mangrove swamps. However, land-use change driven by an expansion in agriculture and logging of trees for timber has reduced the area of mangroves. Moreover, recent offshore explorations for gas have revealed deposits in these parts of the seabed,[39] which may pose an issue in the future, involving trade-offs between hydrocarbon extraction and the ecological security of the region.
Figure 5: Representative Map Identifying the Regions and Zones of the Composite Estuarine and Deltaic Ecosystem
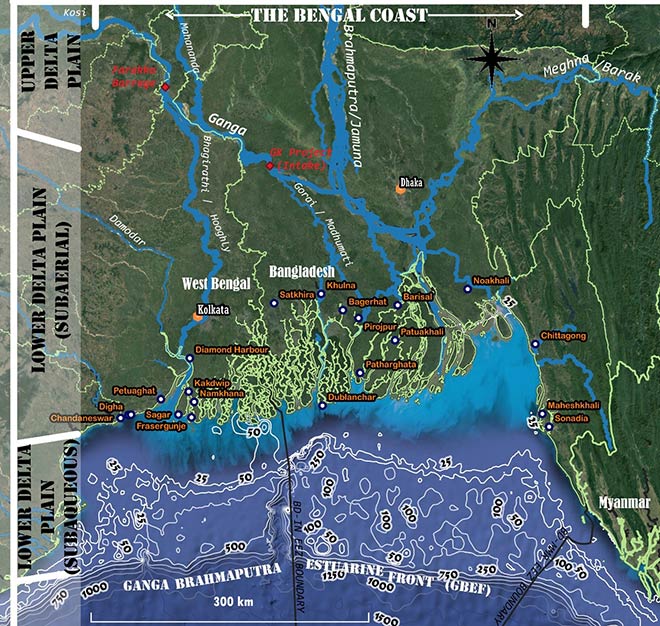
Source: Author’s own
Key Ecosystem Processes
The processes discussed in this section are essential for creating the foundational blocks upon which the ecosystem exists, and any hindrance to these may cause irreversible damage to the ecosystem. Moreover, due to the sheer volume of freshwater and sediment discharge from GBM outlet, the influence extends beyond the coastal waters to encompass the BOBLME. Therefore, these processes are also critical for the overall productivity of the BOBLME.
Supply of Sediments
The GBM system ensures a steady supply of sediments and carries sediments in a suspended state and as bedload. The estimates on the amount of sediment vary. The total water discharge in the Ganga and Brahmaputra ranges from 380 to 590 km3/annum and 400 to 630 km3/annum, respectively. Due to the larger volume of discharge in the Brahmaputra, the sediment load is also higher. The suspended sediment load that is mostly cited for these rivers range from 380 to 480 X 106 tons/annum for the Ganga and 650–680 X 106 tons/annum for the Brahmaputra.[40], [41], [42], [43], [44] While the estimates for the bedload are indistinct, data on Himalayan denudation shows that the sediment export is 50–80 percent higher than the suspended load fluxes.[45], [46] The Ganga and the Brahmaputra have large catchments, drain several geological formations formed during various phases of the earth’s history, and follow two completely different paths to the same outlet. The Meghna originates from a spring in the Manipur hills of India, and much of the river and its tributaries drain the Shillong Plateau and the frontal part of the Indo-Burman Ranges.
The science is fairly clear on the complex relationship between big rivers and coastal fish production. Plumes of freshwater with dissolved nutrients and undissolved sediments (both bedload and suspended) fuel primary productivity of the seas. This, in turn, is food to many aquatic species in the BOBLME. Additionally, the freshwater plumes provide cues to the hilsa to migrate upstream.
Monsoonal rains play a pivotal role in the delivery of freshwater and sediments. Driven by the Southwest monsoon, the maximum discharge for the Ganges and Brahmaputra occurs during June–November and May–November, respectively,[47] which then drops to a minimum during January–April, when the Northeast monsoon takes over. It has been estimated that as much as 80-90 percent of the water discharge in the Ganges occurs during the Southwest monsoon, while the figure is even higher for the Brahmaputra at 95 percent.[48] This indicates that ecosystems in the downstream are attuned to the quantity as well as the timing of the flow. Erodible geological formations in much of the catchment of the rivers, along with strong monsoons, makes the Meghna estuary the largest single-entry point of detritus in the world.[49], [50] This sediment, brought down by the rivers, partly accumulates in the delta and allows for its progradation (See Figure 6). According to estimates, the Ganga supplies 30–40 percent of the sediment in the subaqueous delta and Brahmaputra supplies 60–70 percent. A large part of the net sediment offload bypasses the shelf to generate the turbiditic currents that feed the Bengal-Nicobar Fan, the largest deep-sea cone on the planet.[51]
Figure 6: Location of the GBM Delta and the Movement of Sediments
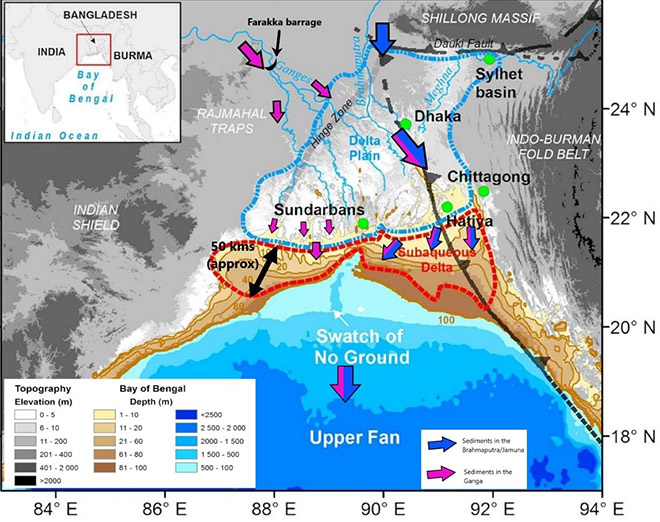
Source: Base map from Karpytchev et al. (2018),[52] modified by the author based on Borromeo et al. (2019).[53]
Tidal Action and Mangroves
Between the open marine waters of the Bay and the mature UDP is an area of fluxes where riverine and marine characteristics converge. This zone mainly comprises tides as the dominant geomorphic agent. The tidal currents allow for the delivery of sediment to form shoals and bars parallel to the flow direction in the river mouth.[54] Over centuries, this action has created the world’s largest tidal delta, the Sundarbans, at 10,000 km2, which is home to a rich variety of flora and fauna that have adapted to the distinctive characteristics of this space. The region is renowned as the most extensive and biologically productive halophytic[g] mangrove forest on earth. Habitats dominated by mangroves serve as breeding ground and nursery for marine life. According to estimates, the effect of mangroves on the annual fish production in India is 1.86 tonnes per hectare, and 23 percent of India’s marine fish output in 2011 can be attributed to mangroves.[55]
The wide spatial and temporal variability in hydrological regimes of freshwater inflow and tides, topography and texture of the substratum, salinity, and their interactions have resulted in a highly heterogenous mangrove ecosystem in this part of the world, evident in the large variety of fish species. The Bangladesh Sundarbans includes 53 pelagic and 124 demersal species of fish,[56], [57] while the Indian Sundarban supports 165 species.[58] Some commercially important species of fish found in brackish water zones of moderate salinity include Tenualosa ilisha, Pomadasys hasta, Polynemus sp. and Coilia sp.[59] Other economically important groups are the crustaceans, such as prawns and shrimps; a substantial proportion of these species are Planktonic.[h] Crabs and lobsters, too, are commercially exploited. Mangroves play an extremely important role as coastal foundation species that modify the habitats of other floral and faunal communities. Without the mangroves, the entire ecosystem in the intertidal zone would collapse.
Water Structure and Oceanography
The East India Coastal Current is the most prominent oceanic circulation in this part of the sea. The current flows in a north-eastward direction along the Indian coast from February to September[60], [61], [62] and then, under the influence of river water exiting into the sea,[63] reverses direction and moves south-eastwards from October to January. However, closer to the coast, the influence of tidal waves is predominant, especially for the inter-tidal zone. Wave height ranges from 1.9 m on the western part to 2.8 m along the eastern part. The gain is primarily because of the funnelling effect of the topography. Further upstream along the rivers that comprise the estuarine part, the height can increase to as much as 5 m.[64] This affects the extent of damage caused by storm surges, when a cyclone overlaps with the occurrence of high tide, thereby impacting the BoB fisheries.
Due to the input of freshwater from the north of the BoB, especially during the monsoons, salinity is the lowest in this part of the sea.[65] The freshwater is then dispersed southwards by the surface currents along the coast. The rainfall during monsoon creates a lot of fluvial runoff to displace marine waters seaward at or near the mouths of the rivers, creating stratification between a layer of freshwater 50–100 m thick on the top and saline water below it.[66] The top layer of mostly freshwater has low salinity and low density, restricting vertical heat transfer between the mixed layer and the thermocline. This keeps the subsurface layer warmer and limits the surface heat loss within the surface layer during winter.[67], [68]
The discharge of sediment-laden freshwater also leads to an increase in chlorophyll (which is a signature for phytoplankton availability) in the river. Strong vertical stratification limits the movement of nutrients from the deeper parts of the sea to the sunlit zone. However, this is not the case near the coast, since coastal currents cause an upwelling of sediments, leading to higher productivity—as evident from the monthly and seasonal variation in Net Primary Productivity (See Figure 7). This is of immense significance, because planktons are the primary producers in the ecological pyramid and many species are dependent on them for their nutritional requirements.[69], [70] A key point to note is that the estuary always has a signature of high chlorophyll concentration, indicating that it remains highly productive throughout the year. Thus, the near-coast region is teeming with marine life, which supports a complex food web and represents rich biodiversity.
Figure 7: Monthly and Seasonal Variation of Net Primary Productivity in the BoB[71]
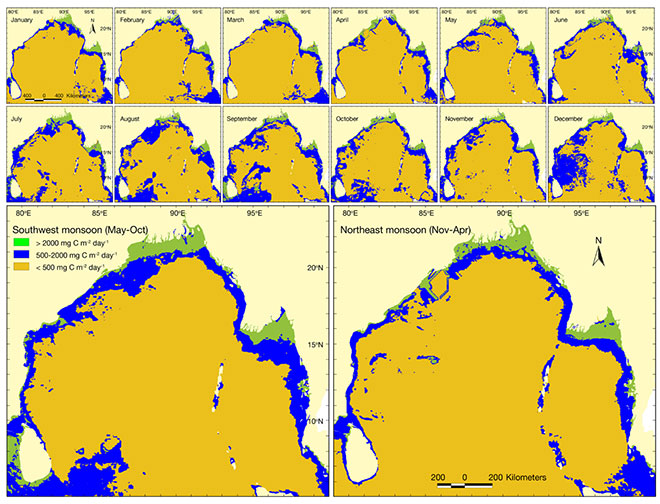
Source: Hossain et al. (2020). Used under Creative Commons 4.0.
Stressors in the Ecosystem
Based on their point/regions of origin, stressors can be categorised as land-based stressors, in-situ stressors, and global stressors. These stressors usually occur in combinations and are responsible for the degradation of the ecosystem in the composite estuarine-deltaic setup.
Land-based
Artificial obstructions along the course of rivers, which impede the free flow of water, has resulted in the trapping of sediments, reduced the depth in river channel during the non-monsoonal months, and altered the annual flow regime of rivers. The diverted water is used to meet the agriculture-water requirements or to provide water to the burgeoning centres of urban growth, e.g. along the Hooghly in West Bengal. This has implications on the quantity, quality and timing of freshwater and sediments reaching the delta. Rahman and Rahman (2018) report the loss in fish diversity due to such human structural interventions, which also disrupt the navigability of a river.[72] Similarly, the ecology of the part of the delta in Bangladesh’s south-west is dependent on the interaction between freshwater supplied by the Gorai-Madhumati channel—a distributary of the Ganga and the saline water from the BoB. In the absence of sufficient freshwater due to the diversion through the canals of the Ganges-Kobadak irrigation project (G-K Project), there has been an increase in salinity in the region, impacting the growth, survival and regeneration of major mangrove plants and as well livelihoods (See Figure 5).[73]
The trapping of sediments poses a particular challenge for the sustenance of deltas in the downstream. Rahman and Rahman estimate that the sediment load in the combined flow of the Ganga and Brahmaputra is decreasing at a rate of 4-10 MT/year. From a previous estimation of mean annual sediment load of 1.0 to 2.4 billion tonnes per year, the sediment load in the GBM system had declined to 500 million tonnes per year in 2015. While this is sufficient to offset the combined effects of land subsidence and sea-level rise, the unsustainable practices in the upstream could soon push the balance below a certain threshold.
Land-based sources of effluents are also emerging as major stressors on the deltaic and estuarine ecosystem. Evidence suggests that there is a progressive decline in the sediment quality of certain creeks within the Sundarbans. The sources of such inorganic contaminants are possibly cargo accidents, port activities, ship breaking, agriculture and aquaculture runoff, etc.[74] The proposed Rampal Power Plant in the periphery of the ecologically fragile Sundarbans landscape could further exacerbate the situation due to its inadequate capacity of dealing with ash disposal.[75]
Other land-based stressors include deterioration of water quality[76], [77], [78] and large-scale land-use change, such as deforestation of mangrove forests.[79], [80]
In-Situ
Primarily of two kinds, these stressors influence the aquatic ecosystem directly. The first kind is the unregulated and often illegal harvest of marine resources. For example, the sharks, skates, and rays in the Indian Sundarbans are overexploited for their body parts such as meat, liver, fins and skin.[81] Similarly, noncompliance of rules with regard to the prohibition of small mesh size (< 60mm) results in the frequent capture of juvenile fish of commercially valuable species such as hilsa. Fishers also capture the female hilsa at the mouth of the estuary during upstream migration for breeding, adversely affecting population recruitment.[82]
The second kind of in-situ stressor is the toxic spill of fly ash in the estuarine waters of the Sundarbans. Annually, India exports three million tonnes of fly ash to Bangladesh, where it is used in the cement industry.[83] Fly ash is highly toxic and contains various lethal components such as lead, arsenic, mercury, cadmium and uranium. The environmental impact of these accidental spills is immense, yet they remain understudied from the perspecive of their impact on the fragile Sundarbans ecosystem.
Global
These stressors can be mostly attributed to a changing climate, which create a cascading impact on the ecosystem of the region. Coastal areas in the GBM delta are already experiencing a sea-level rise of 5mm/year.[84], [85] Land subsidence due to active tectonics and other factors further add to the ordeals of the delta. The sea-level rise to this local subsidence has been estimated to be 3mm/year.[86] Furthermore, sea temperature is also set to rise by 1-2 °C by the middle of this century and 2-3 °C by the end of the century, in a scenario of medium greenhouse gas emissions.[87] Changes in sea temperature can further cause stronger stratifications and reduce nutrient flow to the surface of the water, lowering the productivity of the ecosystem as a whole. Increased sea surface temperatures also fuel cyclone intensity and contribute to increased storm surges in the coastal regions and along the creeks, which can destroy the mangrove-based ecosystem in the inter-tidal zone.
Due to the continuity of the region to the land and the resulting interconnectedness, the impact of climate change on land and measures taken in response can significantly affect the estuarine and deltaic ecosystem. For example, most simulation models indicate a wetter climate for the Ganga and Brahmaputra basin in the future,[88] and an increase in both intensity and frequency of extreme precipitation.[89] This might lead to greater sediment load in the rivers, which is also dependent on human activities on land and along river courses.
India-Bangladesh Cooperation for EAFM
The idea that ecosystems must be the basic unit of governance is not new. Ecosystem management is an area-based approach, with boundaries clearly and formally defined to maintain ecosystems in the condition best-suited for achieving desired social benefits. Moreover, the focus of such management must be the state of the ecosystem (health, integrity) rather than the system output, while restoring critical ecological components, functions and structures to sustain resources in perpetuity.[90] In this regard, society plays a critical role in deciding the desired state of the ecosystem for management. Thus, it is important to actively involve local stakeholders, i.e. the fisherfolk, since their livelihood depends on the state of the ecosystem.
A conventional approach to fisheries management focuses on maximising the economic benefits while ensuring that the catch is commensurate with the natural productivity of the harvest stocks.[91] This is inherently narrow in its scope and focuses on fishing activity that affects single or target stocks. An ecosystem approach to fisheries is a way to incorporate ecosystem considerations into more conventional fisheries management, accomodating a broad ambit of governance goals[i] such as development, planning, and food safety. The EAFM is aimed at planning, developing and managing fisheries in a manner that addresses the multiplicity of societal needs and wants, without jeopardising the options for future generations to benefit from a full range of goods and services provided by marine ecosystems.[92] Thus, the EAFM will foster consensus-based management, which is both well-coordinated and focused on sustainability.
Based on the shared stock of marine fish resources as well as the jurisdiction over parts of a single ecosystems (discussed in previous sections), cooperation between India and Bangladesh is essential.[j] Among the land-based stressors (such as impoundments on river systems from the mountains to the sea, plastic pollution etc.), some are transboundary in nature. Moreover, some in-situ stressors, such as toxic spills of fly ash and the transgressions by fishers in search of better catch, can become issues of high-stake and diplomatic concern between the two nations. as was the case regarding hydrocarbons before the demarcation of the Exclusive Economic Zone (EEZ). Thus, transboundary cooperation is the only way forward.
The following are the potential areas of cooperation between India and Bangladesh under the paradigm of EAFM.
- The holistic management of fisheries demands an approach that is not confined by international borders on the sea. Therefore, the first and foremost goal should be to adopt EAFM, through which the contiguous stretches of the sea, under different possessions, would be adopted as one composite unit of governance. A starting point is to reach a common understanding of what constitutes the habitat of the commercially valuable marine species, the broad ecosystem of which they are a part, and the natural processes that are essential for the sustenance of the ecosystem. This will facilitate cooperation between the two nations for conserving the stock of commercially valuable marine species and ensuring that the ecosystem remains in a healthy state. At the same time, the all-evident cost of non-cooperation and the inevitable decline in fish catch resulting from overexploitaion can also foster cooperation. It is also important to emphasise that various stakeholders, and not only nations, must collaborate within and across the nations and agree upon a desired state of the ecosystem. Thereafter, action plans should be devised along with the bolstering of institutional capacities to implement such plans in a coordinated manner. The science of EAFM is still emerging and improving and so is the understanding of the nature of threats. Therefore, such plans need to incorporate the ‘best available science’ for the holistic management of fisheries.
- Sediment-laden freshwater inflow into the delta appears to be the primary concern for restoring the habitat of various commercially valuable marine species such as the hilsa. As discussed in this paper, the catch of hilsa has gradually reduced in the Hooghly-Bhagirathi. Therefore, driven by a continuously growing demand for the fish, India has become heavily dependent on hilsa imports from Bangladesh and Myanmar. Bangladesh has already realised the value of the lucrative industry of hilsa fisheries on which millions depend for their livelihood, and has taken proactive steps to regulate harvests. India, or more specifically West Bengal,[k] seems to be be lagging in this respect. The poor state of regulation that does not involve the community is only a reflection of the issues that need to be tackled. The two nations must devise a comprehensive water and sediment management plan to best emulate the natural processes in the delta and the ecosystem. Contentious but important issues such as the fate of the decades-old Farakka Barrage must be deliberated between the governments of the two countries while also involving the state government of West Bengal. In the build-up to the post-2026 arrangement, following the end of validity of the Ganges Water Sharing Treaty, it is important that neither country gets entangled in the binary of choices, i.e. keeping or dismantling the barrage. Water requirement for stable ecosystems should also feature prominently in such dialogues.
- The mangrove ecosystem of the Sundarbans, with its floral and faunal diversity, is critical for both inland and marine fisheries in India and Bangladesh. In 2011, India and Bangladesh signed a Memorandum of Understanding (MoU) on “Conservation of the ” The MoU envisages joint action ranging from delineating the region to developing a shared understanding of the impacts of climate change and addressing issues of livelihood of the ecosystem-dependent population. However, progress has been confined to the joint assessment of tigers and the remaining areas of action remain unexplored, e.g. carbon sequestration, maintaining floral and faunal diversity, and reducing the direct and indirect anthropogenic pressure. Danda (2019) notes even with a vigorous persuasion of the mandates such as mitigating coastal flooding due to sea-level rise, halting or reversing erosion, ensuring sediment supply, recovering the damages, and facilitating the inland movement of mangroves, little success will be achieved since the work is beyond the purview of the Forest Department in the two countries and unilateral interventions might not yield desired results. Thus, external assistance in terms of technology and finance will be critical.[93] It is in the immediate interest of both countries to increase their focus on this aspect, to prevent the degradation of the Sundarbans and ensure that the deltaic complex can continue to perform the role of a nursery for the BoB fisheries.
- India and Bangladesh must undertake joint transboundary stock assessment, involving cost-sharing and inputs from experts from both countries. So far, no assessment has been done that conclusively identifies the volume of the stock, and proxies such as CPUE are readily used. Without a clear understanding of the stock of the shared resource along with a mutual consensus, all initiatives to conserve the ecosystem will be based on estimates, which might jeopardise the long-term plans while instilling little confidence, if any. An MoU signed between India and Bangladesh in 2011 regarding cooperation in the field of fisheries already has provisions for joint activities and programmes, and the exchange of scientific materials, information and personnel (Article 1). It also mentions Hilsa fisheries management amongst a few other activities (Article 2). However, the MoU does not include joint exercises for stock assessment, mentioning instead only “training in fish stock assessment.”
- To ensure inclusivity, the local governments and fishing communities must be made stakeholders. The EAFM calls for a participatory approach, wherein society must decide in a collective manner regarding the state of the ecosystem that they desire. The fisherfolk are the primary stakeholders in this scenario, since their livelihood is directly dependent on the health of the industry. This process will require their active involvement from across borders to arrive at mutually agreed goals and to devise appropriate rules. This will ensure greater compliance, since the rules will be outlined through a consultative process with the active participation of those responsible for upholding them. The involvement of targeted Information, Education and Communication (IEC) campaigns for creating awareness and consensus for collective action would also be critical.
Conclusion
It is in the best interest of India and Bangladesh to create an enabling framework for the realisation of EAFM in the contiguous waters off the Bengal Coast. The conservation of the ecosystem is a human need and this cannot be emphasised any further in the context of coastal fisheries. Ecosystems will have to be protected if the industry around marine fisheries has to continue—in this, there is a clear economic case.
The collapse of fisheries, most notably the hilsa, will spell doom for the millions of fishers in West Bengal and Bangladesh who are dependent on this industry for their livelihood. The need to secure these livelihoods should also pave the way for stronger political will and concerted, collaborative action to safeguard the shared interests of the two nations. Moreover, a framework for cooperative federalism between West Bengal and the Union Government in India will be key to achieving progress.
About the Author
Sayanangshu Modak is Junior Fellow at ORF, Kolkata.
Endnotes
[a] It is an outlet of the mighty Ganga Brahmaputra Meghna (GBM) river system (the third-largest river system on the planet in terms of freshwater discharge and sediment contribution) and is underlain by the subaqueous section of the world’s largest delta – the Bengal Delta.[a]
[b] Dutta et al. (2019) calculated the rate of exploitation of the hilsa shad in the estuarine waters of the Sundarbans of India and found it to be 0.78 while Nurul Amin et al. (2008) found it to be 0.66 in the coastal waters of Bangladesh. A value of more than 0.50 means the fish is overharvested from a particular area and during some specific period.
[c] Clinoforms are inclined striatal surfaces that occur over various spatial scales, ranging from delta‐front forests to continental margin slopes.
[d] In sedimentary geology and geomorphology, the term ‘progradation’ refers to the growth of a river delta farther out into the sea over time.
[e] Meaning “above water.”
[f] Avulsions are the natural processes by which flow diverts out of an established river channel into a new permanent course on the adjacent floodplain abandoning the former channel.
[g] A salt-tolerant plant that grows in soil or waters of high salinity, coming into contact with saline water through its roots or by salt spray.
[h] They consume planktons as food.
[i] Ref. to the consensus reached at the Reykjavik Conference in 2002
[j] For the case of the Hilsa, the stock is shared between India, Bangladesh and Myanmar and provides scope for trilateral cooperation. However, for this study, the focus has been India and Bangladesh owing to not just their proximity but also the contiguity of ecological features.
[k] Since fisheries is a state subject, fishing in the Internal Waters, Territorial Sea and Rivers come within the purview of the state government.
[1] Ministry of Fisheries, Animal Husbandary and Dairying, Handbook on Fisheries Statistics 2018, September 2019, New Delhi, Fishery Survey of India, 2019.
[2]Ministry of Fisheries and Livestock, Yearbook of Fisheries Statistics of Bangladesh 2017-18, December 2018, Dhaka, DG Department of Fisheries, 2018.
[3] D.K. De, and N.C. Dutta, “Studies on certain aspects of the morpho-histology of Indian Shad Hilsa, Tenualosa ilisha (Hamilton) in relation to food and feeding habits,” Indian Journal of Fisheries 37(3) (1990): 189–198.
[4] S. Reuben et al., “The resources of Hilsa Shad, Hilsa ilisha (Hamilton), along the Northeast coast of India,” Indian Journal of Fisheries 39 (3&4) (1992): 169–181.
[5]M.J. Rahman and I.G. Cowx,“Population dynamics of Hilsa Shad (Tenualosa ilisha, Clupeidae) in Bangladesh waters,” Asian Fisheries Science 21 (2008):85–100.
[6] Amin Nurul et al., “Population dynamics and stock assessment of Hilsa Shad, Tenualosa ilisha in Bangladesh,” Asian Fisheries Science 15 (2002): 123–128.
[7] Nurul Amin et al., “Stock assessment and management of Tenualosa ilisha in Bangladesh,” Asian Fisheries Science 17 (2004): 51–59,
[8] Protection and Conservation of Fish Act, 1950 (E.B. Act XVIII of 1950)
[9] S. Ghosh, “The Iconic Hilsa Is Facing a Threat of Being Fished out of West Bengal Rivers,” Mongabay, May 7, 2018.
[10] Government of West Bengal, Fisheries Department, Department notification no. 1979-Fish/C-1 (Kolkata: 2013) https://smallscalefishworkers.org/wp-content/uploads/2019/01/Hilsa-Notification-inland.pdf
[11] Mohammad Mahmudul Islam et al., “Fisheries Co-Management in Hilsa Shad Sanctuaries of Bangladesh: Early Experiences and Implementation Challenges,” Marine Policy 117 (2020): 103955, https://doi.org/10.1016/j.marpol.2020.103955
[12] Ray Hilborn and Carl J. Walters,Quantitative Fisheries Stock Assessment: Choice, dynamics and uncertainty (Dordrecht: Springer Science & Business Media, 1992), pp. 104–155.
[13] T.J.Ii. Quinn & R.B. Deriso, Quantitative Fish Dynamics. (New York: Oxford University Press, 1999), pp. 542.
[14] Jens Ambsdorf et al., ,Ocean Atlas. Facts and Figures on the Threats to Our Marine Ecosystems, (Paderborn: Bonifatius GmbH Druck – Buch – Verlag,2017)pp.42-43.
[15] M. Das. “How much do Bengalis crave Bangladeshi hilsa? Enough to smuggle 2,800 kg during pandemic.” The Print, September 5, 2020.
[16]Hossain et al.,” Tropical hilsa shad (Tenualosa ilisha): Biology, fishery and management”
[17] M. S. Hossain et al.,”Habitats across the life cycle of Hilsa shad (Tenualosa ilisha) in aquatic ecosystem of Bangladesh,” Fisheries Management and Ecology 23, no. 6 (2016): 450-462.
[18] M.S. Hossain et al., “Primary productivity connects hilsa fishery in the Bay of Bengal.” Scientific Reports 10 (2020): 1-16, https://doi.org/10.1038/s41598-020-62616-5
[19] MA Mome and R. Arnason, The potential of the artisanal Hilsa fishery in Bangladesh: An economically efficient fisheries policy, United Nations University Fisheries Training Program, 2007.
[20] Central Marine Fisheries Research Institute, Annual Report 2010-2011. Kerala, India, 2011.
[21] Wahab MA, Beveridge MCM and Phillips MJ,eds., Hilsa: Status of fishery and potential for aquaculture (Penang, Malaysia: WorldFish, 2019), Proceedings: 2019-16, 196p.
[22] Department of Fisheries (DoF), Fisheries Statistical Yearbook of Bangladesh 2006-07, 2008.
[23] BOBLME,Report of the hilsa fisheries assessment working group meeting, November 2014, Kolkata, India, Bay of Bengal Large Marine Ecosystem Project http://aquaticcommons.org/19208/1/BOBLME-2014-Ecology-12.pdf
[24] “Hilsa: Status of fishery and potential for aquaculture”
[25] “Hilsa: Status of fishery and potential for aquaculture”
[26]M J Rahman et al., “Hilsa Fishery Management in Bangladesh.” IOP Conference Series: Earth and Environmental Science 414 (2020): 012018, https://doi.org/10.1088/1755-1315/414/1/012018
[27] Milan Datta, “Laws Flouted, Hilsa Dies in West Bengal,” The Third Pole, November 27, 2018.
[28] A. Bladon, “Cooperation vs. Competition over Shared Fish Stocks,” International Institute for Environment and Development, April 23, 2019. https://www.iied.org/cooperation-vs-competition-over-shared-fish-stocks.
[29] Utpal Bhaumik, “Fisheries of Indian Shad (Tenualosa Ilisha) in the Hooghly–Bhagirathi Stretch of the Ganga River System,” Aquatic Ecosystem Health & Management 20, no. 1-2 (2017): 130–139.
[30] Utpal Bhaumik et al., “Impact of hydro-ecology on fish migration in selected east coast estuaries.” J. Inland Fish. Soc. India 43, no. 1 (2011): 1-9.
[31] Bhaumik.,“Fisheries of Indian Shad (Tenualosa Ilisha) in the Hooghly–Bhagirathi Stretch of the Ganga River System”
[32] Ahsan Dewan Ali et al., “Migration, spawning patterns and conservation of Hilsa Shad (Tenualosa ilisha) in Bangladesh and India.” Published by Academic Foundation India, New Delhi and International Union for Conservation of Nature and Natural Resources (IUCN) 95 (2014).
[33] BOBLME, Procedures and methods for continuing assessment of the status of Hilsa resources in India, 2015, Kolkata, India, Bay of Bengal Large Marine Ecosystem Project.
[34] FAO/GEF, Sustainable Management of the Bay of Bengal Large Marine Ecosystem (BOBLME).
[35] Heileman, S., G. Bianchi, and S. Funge-Smith,The UNEP large marine ecosystems: A perspective on changing conditions in LMEs of the world’s regional seas, Nairobi, United Nations Environment Programme, 2009, https://iwlearn.net/resolveuid/848c0f306710edd035e8cfe6e7cd1def
[36] A. Dai and K.E. Trenberth, “Estimates of freshwater discharge from continents: Latitudinal and seasonal variations,” Journal of Hydrometeorology 3, (2002) https://doi.org/10.1175/1525-7541(2002)003%3C0660:EOFDFC%3E2.0.CO;2
[37] Steven A. Kuehl et al., “The Ganges–Brahmaputra Delta.” in River Deltas-Concepts, Models, and Examples, ed. Liviu Giosan, Janok P. Bhattacharya (SEPM Society for Sedimentary Geology,2005), 413–34, https://doi.org/10.2110/pec.05.83.0413
[38] Kuehl et al., “The Ganges–Brahmaputra Delta,”413–34.
[39] Ian Blakely, “Bay of Bengal: Many Possibilities and Challenges Ahead,” GEOExPro, 2010.
[40] Maarten Lupker et al., “10Be-Derived Himalayan Denudation Rates and Sediment Budgets in the Ganga Basin,” Earth and Planetary Science Letters 333-334 (2012): 146–56, https://doi.org/10.1016/j.epsl.2012.04.020
[41] Maarten Lupker et al., “Be Systematics in the Tsangpo-Brahmaputra Catchment: The Cosmogenic Nuclide Legacy of the Eastern Himalayan Syntaxis.” Earth Surface Dynamics 5, no. 3 (2017): 429–49.
[42] Delft Hydraulics, River Survey Project, Flood Action Plan 24, Special Report n°18. In Sediment Rating Curves and Balances,Dhaka, Bangladesh, Water Resources Planning Organization, 1996
[43] William W. Hay. “Detrital Sediment Fluxes from Continents to Oceans.” Chemical Geology 145, no. 3-4 (1998): 287–323, https://doi.org/10.1016/S0009-2541(97)00149-6
[44] Mohammad Rezwanul Islam et al., “The Ganges and Brahmaputra Rivers in Bangladesh: Basin Denudation and Sedimentation.” Hydrological Processes 13, no. 17 (1999), https://doi.org/10.1002/(SICI)1099-1085(19991215)13:17%3C2907::AID-HYP906%3E3.0.CO;2-E
[45] Maarten Lupker et al., “10Be-Derived Himalayan Denudation Rates and Sediment Budgets in the Ganga Basin”
[46] Maarten Lupker et al., “Be Systematics in the Tsangpo-Brahmaputra Catchment: The Cosmogenic Nuclide Legacy of the Eastern Himalayan Syntaxis.”
[47] James M. Coleman, “Brahmaputra River: Channel Processes and Sedimentation.” Sedimentary Geology 3, no. 2-3 (1969), https://doi.org/10.1016/0037-0738(69)90010-4
[48] V. Subramanian and A. L. Ramanathan, “Nature of Sediment Load in the Ganges-Brahmaputra River Systems in India” in Sea-Level Rise and Coastal Subsidence. Coastal Systems and Continental Margins, ed. J.D. Milliman and B.U. Haq (Dordrecht: Springer, 1996), 101–2.
[49] John D. Milliman, and James P. Syvitski, “Geomorphic/Tectonic Control of Sediment Discharge to the Ocean: The Importance of Small Mountainous Rivers.” The Journal of Geology 100, no. 5 (1992): 525–44.
[50] Steven L. Goodbred, and Steven A. Kuehl. “Enormous Ganges-Brahmaputra Sediment Discharge during Strengthened Early Holocene Monsoon.” Geology 28, no. 12 (2000): 1083. https://doi.org/10.1130/0091-7613(2000)28%3C1083:EGSDDS%3E2.0.CO;2
[51] Joseph R. Curray et al., “The Bengal Fan: Morphology, Geometry, Stratigraphy, History and Processes.” Marine and Petroleum Geology 19, no. 10 (2003): 1191–1223, https://doi.org/10.1016/S0264-8172(03)00035-7
[52] M. Karpytchev et al., “Contributions of a Strengthened Early Holocene Monsoon and Sediment Loading to Present‐Day Subsidence of the Ganges‐Brahmaputra Delta.” Geophysical Research Letters 45, no. 3 (2018): 1433–42, https://doi.org/10.1002/2017GL076388
[53]Laura Borromeo et al., “Provenance of Bengal Shelf Sediments: 1. Mineralogy and Geochemistry of Silt.” Minerals 9, no. 10 (2019): 640, https://doi.org/10.3390/min9100640
[54] Kimberly G. Rogers and Steven L. Goodbred, “The Sundarbans and Bengal Delta: The World’s Largest Tidal Mangrove and Delta System.” in Landscapes and landforms of India, ed. V. Kale (Dordrecht: Springer, 2014), 181-87
[55] Anneboina, Lavanya Ravikanth, and KS Kavi Kumar. “Economic analysis of mangrove and marine fishery linkages in India.” Ecosystem services 24 (2017), https://doi.org/10.1016/j.ecoser.2017.02.004
[56] S. U. Sarkar, “Fish Eating Wildlife and Some Fishes of the Sundarbans, Bangladesh.” The Journal of Noami, 6(1-2), (1989): 17–29.
[57] Walter J. Rainboth.“The fish communities and fisheries of the Sundarbans with a framework for future studies” in The Commons in South Asia: Societal Pressures and Environmental Integrity in the Sundarbans, ed. Seidensticker, R. Kurin, and A. K. Townsend (Washington D.C., The International Center, Smithsonian Institution, 1991)
[58]P. Sanyal, “Sundarbans – the largest mangrove diversity on globe” in Sundarbans Mangal, ed.D. N. Guha Bakshi, P. Sanyal and K. R. Naskar (Calcutta,Naya Prokash, 1991), 428–448
[59] Brij Gopal and Malavika Chauhan. “Biodiversity and Its Conservation in the Sundarban Mangrove Ecosystem,” Aquatic Sciences 68, no. 3 (2006): 338–54.
[60] A. V. Chaitanya et al., “Salinity Measurements Collected by Fishermen Reveal a ‘River in the Sea’ Flowing Along the Eastern Coast of India,” Bulletin of the American Meteorological Society 95, no. 12 (2014)https://doi.org/10.1175/BAMS-D-12-00243.1
[61] Durand et al., “Spatiotemporal structure of the East India coastal current from satellite altimetry,” Journal of Geophysical Research-Oceans 114 (2009), https://doi.org/10.1029/2008JC004807
[62] D. Shankar et al., Dynamics of the east India coastal current: Analytic solutions forced by interior Ekman pumping and local alongshore winds. Journal of Geophysical Research-Oceans 101 (C6)(1996): 13975–13991
[63] N.A. Diansky et al., High resolution modelling of the monsoon circulation in the Indian Ocean. Oceanology 46 no. 5 (2006), https://doi.org/10.1134/S000143700605002X
[64] Dilip K. Barua. “Suspended Sediment Movement in the Estuary of the Ganges-Brahmaputra-Meghna River System.” Marine Geology 91, no. 3 (1990): 243–53.
[65] Vimlesh Pant et al., “Observed Interannual Variability of near-Surface Salinity in the Bay of Bengal,” Journal of Geophysical Research: Oceans 120, no. 5 (2015), https://doi.org/10.1002/2014JC010340
[66] Clifford S. Felton et al., “Estimation of the Barrier Layer Thickness in the Indian Ocean Using Aquarius Salinity,” Journal of Geophysical Research: Oceans 119, no. 7 (2014), https://doi.org/10.1002/2013JC009759
[67] Pankajakshan Thadathil et al., “Surface Layer Temperature Inversion in the Bay of Bengal,” Deep Sea Research Part I: Oceanographic Research Papers 49, no. 10 (2002), https://doi.org/10.1002/2016JC011674
[68] Thompson et al.,.”Seasonal evolution of temperature inversions in the north Indian Ocean,” Current Science 90(5) (2006), 697–704
[69] R. K. Mishra et al., “Seasonal Appearance of Chlorophyceae Phytoplankton Bloom by River Discharge off Paradeep at Orissa Coast in the Bay of Bengal,” Environmental Monitoring and Assessment 149, no. 1-4 (2008), https://doi.org/10.1007/s10661-008-0200-2
[70] Anup K. Prasad and Ramesh P. Singh, “Chlorophyll, Calcite, and Suspended Sediment Concentrations in the Bay of Bengal and the Arabian Sea at the River Mouths,” Advances in Space Research 45, no. 1 (2010), https://doi.org/10.1016/j.asr.2009.07.027
[71] Hossain et al., “Primary productivity connects hilsa fishery in the Bay of Bengal.”
[72] Md. Mahbubur Rahman and Muhammad Mizanur Rahaman. “Impacts of Farakka Barrage on Hydrological Flow of Ganges River and Environment in Bangladesh.” Sustainable Water Resources Management 4, no. 4 (2017), https://doi.org/10.1007/s40899-017-0163-y
[73] Monirul M. Mirza,The Ganges Water Diversion: Environmental Effects and Implications — An Introduction by Monirul M. Mirza eds. The Ganges Water Diversion: Environmental Effects and Implications,(Water Science and Technology Library, vol 49. Springer, Dordrecht, 2004),1-12
[74]M.A., Islam et al., “Contamination and Ecological Risk Assessment of Trace Elements in Sediments of the Rivers of Sundarban Mangrove Forest, Bangladesh,” Marine Pollution Bulletin 124, no. 1 (2017) https://doi.org/10.1016/j.marpolbul.2017.07.059
[75] A. Dennis. Lemly. “Environmental Hazard Assessment of Coal Ash Disposal at the Proposed Rampal Power Plant,” Human and Ecological Risk Assessment: An International Journal 24, no. 3 (2018) https://doi.org/10.1080/10807039.2017.1395685
[76] P. G. Whitehead et al., “Impacts of Climate Change and Socio-Economic Scenarios on Flow and Water Quality of the Ganges, Brahmaputra and Meghna (GBM) River Systems: Low Flow and Flood Statistics,” Environmental Science: Processes & Impacts 17, no. 6 (2015) https://doi.org/10.1039/c4em00619d
[77] P. G. Whitehead et al., “Dynamic Modeling of the Ganga River System: Impacts of Future Climate and Socio-Economic Change on Flows and Nitrogen Fluxes in India and Bangladesh.” Environmental Science: Processes & Impacts 17, no. 6 (2015) https://doi.org/10.1039/c4em00616j
[78] L. Jin et al., “Assessing the Impacts of Climate Change and Socio-Economic Changes on Flow and Phosphorus Flux in the Ganga River System,” Environmental Science: Processes & Impacts 17, no. 6 (2015) https://doi.org/10.1039/c5em00092k
[79] A. Mukhopadhyay et al., “Dynamics of the Sundarbans Mangroves in Bangladesh Under Climate Change” in Ecosystem Services for Well-Being in Deltas, ed. Nicholls et al.(Palgrave Macmillan, Cham, 2018), 489-503
[80]Anirban Mukhopadhyay et al., “Changes in Mangrove Species Assemblages and Future Prediction of the Bangladesh Sundarbans Using Markov Chain Model and Cellular Automata,” Environmental Science: Processes & Impacts 17, no. 6 (2015) https://doi.org/10.1039/C4EM00611A
[81] Mousumi Pal et al., “An overview of the fishes of Indian Sundarbans and their conservation status.” Journal of Environment and Sociobiology 11, no. 2 (2014): 171-186.
[82] Ahsan Dewan Ali et al., Migration, spawning patterns and conservation of Hilsa Shad (Tenualosa ilisha) in Bangladesh and India(New Delhi: Academic Foundation India,2014) https://portals.iucn.org/library/node/45753.
[83] Namrata Acharya, “Toxic Spillover: The Unhindered Fly Ash Export from India To Bangladesh,” The Wire Science, October 26, 2020.
[84] Charls Antony et al., “Evolution of Extreme High Waters along the East Coast of India and at the Head of the Bay of Bengal,” Global and Planetary Change 140 (2016) https://doi.org/10.1016/j.gloplacha.2016.03.008
[85] A.S. Unnikrishnan and D. Shankar, “Are Sea-Level-Rise Trends along the Coasts of the North Indian Ocean Consistent with Global Estimates?” Global and Planetary Change 57, no. 3-4 (2007): 301–7.
[86] S. Brown and R.J. Nicholls. “Subsidence and Human Influences in Mega Deltas: The Case of the Ganges–Brahmaputra–Meghna,” Science of The Total Environment 527-528 (2015) https://doi.org/10.1016/j.scitotenv.2015.04.124
[87] Jose A. Fernandes et al., “Projecting Marine Fish Production and Catch Potential in Bangladesh in the 21st Century under Long-Term Environmental Change and Management Scenarios,” ICES Journal of Marine Science 73, no. 5 (2015) https://doi.org/10.1093/icesjms/fsv217
[88] P F. Uhe et al., “Enhanced Flood Risk with 1.5 °C Global Warming in the Ganges–Brahmaputra–Meghna Basin,” Environmental Research Letters 14, no. 7 (2019) https://doi.org/10.1088/1748-9326/ab10ee.
[89] Amulya Chevuturi et al., “Projected Changes in the Asian‐Australian Monsoon Region in 1.5°C and 2.0°C Global‐Warming Scenarios,” Earth’s Future 6, no. 3 (2018) https://doi.org/10.1002/2017EF000734
[90] H.J. Cortner et al., “Institutional Barriers and Incentives for Ecosystem Management: A Problem Analysis.,” no. 16 (Tucson, University of Arizona, Water Resources Research Centre, 1994), pp. 51.
[91]“Why Do We Need to Use an Ecosystem Approach to Fishery …”.
[92] FAO. “The ecosystem approach to marine capture fisheries. FAO Technical Guidelines for Responsible Fisheries”, no. 4 (2003), pp. 112.
[93] Anamitra Anurag Danda, “Environmental Security in the Sundarban in the Current Climate Change Era: Strengthening India-Bangladesh Cooperation,” Occassional Paper, No. 220, (Observer Research Foundation, 2019 )
The views expressed above belong to the author(s). ORF research and analyses now available on Telegram! Click here to access our curated content — blogs, longforms and interviews.

 PDF Download
PDF Download










 PREV
PREV


