Introduction
In India, cardiovascular diseases (CVDs) have now become the leading cause of mortality, with a quarter of all deaths in 2015 attributed to the disease. According to the Global Burden of Disease (GBD) 2015 study, the age-standardised CVD death rate for India stands at 272 per 100,000 population, although other recent, large prospective studies have shown a higher age-standardised CVD mortality rate (225-525 per 100,000 in men and 225-299 per 100,000 in women).[i] Even with such figures, CVD in India remains highly under-diagnosed though the number of Percutaneous Coronary Interventions (PCIs)[ii] is growing every year. Estimates from the National Intervention Council (NIC) Registry of the Cardiology Society of India (CSI) show that the number of catheterisation laboratories[iii] in the country has risen from 251 in 2010 to 630 in 2015, with a 51-percent increase in total coronary interventions between 2014 and 2015. In 2015, 475,000 stents were used for 375,000 coronary interventions, or an average of 1.27 per procedure (Table 1).
Table 1: Details of cath-labs, coronary interventions and stents, 2010-2015
|
2010 |
2011 |
2012 |
2013 |
2014 |
2015 |
| Cath-lab Centres |
251 |
332 |
369 |
404 |
396 |
630 |
| Coronary interventions |
117420 |
152332 |
177240 |
216817 |
248152 |
375000 |
| Coronary interventions per centre |
468 |
459 |
480 |
537 |
627 |
595 |
| Total stents |
146719 |
193728 |
215662 |
262349 |
248152 |
475000 |
| Stents per procedure |
1.25 |
1.27 |
1.22 |
1.21 |
1.00 |
1.27 |
Source: Estimates compiled by authors from various presentations and reports made by the National Intervention Council over the years[iv]
The recent order by the National Pharmaceutical Pricing Authority (NPPA), F.No. 19(837)/2016/Div.II/DP/NPPA, dated 2 February 2017, fixed a ceiling on the price of cardiac stents. In issuing its order, the NPPA stressed that margins have become exorbitant and irrational, and profiteering was rampant at various levels in the supply chain including the private hospitals and cardiology clinics. NPPA said these raised various economic and ethical arguments in the health financing sphere. The order also states that the levels of margins indicate a “failed market system” where asymmetry of information has resulted in unethical practices.[v]
In December 2014, lawyer Birendra Sangwan took up the battle on the exorbitant pricing of cardiac stents by filing a query under the Right to Information Act. Sangwan challenged the NPPA and referred to the case of a friend’s father who was charged a massive INR 1.20 lakh for his cardiac stents by a Faridabad-based private hospital. In its reply, the NPPA said that since stents were notified as ‘drugs’ under the Drugs and Cosmetics Act and are not part of the National List of Essential Medicines (NLEM), no ‘ceiling price’ was fixed on them.
Sangwan went on to file a PIL in 2015. Initially, the government did not respond to the High Court’s order to include stents in the NLEM and set a cap on their prices. Only after a petition for contempt was filed in July 2016 did the government in December that year include stents in the first Schedule of the Drug Prices Control Order. Finally, in February 2017, the prices of stents were capped at INR 7,260 for bare metal stents (BMS), and INR 29,600 for drug eluding stents (DES).[vi] Before that, the average retail price for a bare metal stent was INR 45,000, while drug-eluding stents were priced at around INR 1.2 lakh, generating profit margins that ranged from 270 percent to 1,000 percent.[vii] The government then took credit for the massive slash in the price of cardiac stents.[viii]
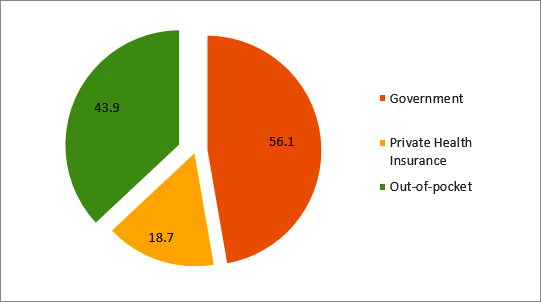
It is noteworthy that the share of DES in the total use of stents has been steadily increasing: a CAGR of 53.52 percent compared to 22.86 percent for total stents for the period 2002 to 2015 (Figure 1). Recent studies have shown that the clinical effectiveness of DES in comparison with that of BMS is not significantly different, and is cost-effective only among patients with specific risks. A 2016 randomised controlled trial involving 9,013 patients showed that for patients undergoing percutaneous coronary intervention (PCI), at six years, no significant differences between those receiving drug-eluting stents and those receiving bare-metal stents in the composite outcome of death from any cause and non-fatal spontaneous myocardial infarction were observed.[ix] With regards to cost-effectiveness, a 2012 systematic review of cost-effectiveness studies comparing DES vs. BMS found DES to be cost-effective only in studies reporting a greater risk of restenosis or with multiple vessel disease.[x] This evidence has to be seen in the light of the fact that over 43.9 percent of the financing of the coronary procedures in 2014 in India were conducted through out-of-pocket expenditure (see Figure 2). According to the analysis of the NSSO 71st round, one-fifth of hospitalisations due to CVD were paid for by borrowings or sale of personal assets. The same survey found that 53 percent of the population suffered from ‘catastrophic’ health expenditures.[xi]
Figure 1: Usage patterns of stents and drug eluting stents (DES) in India [xii]
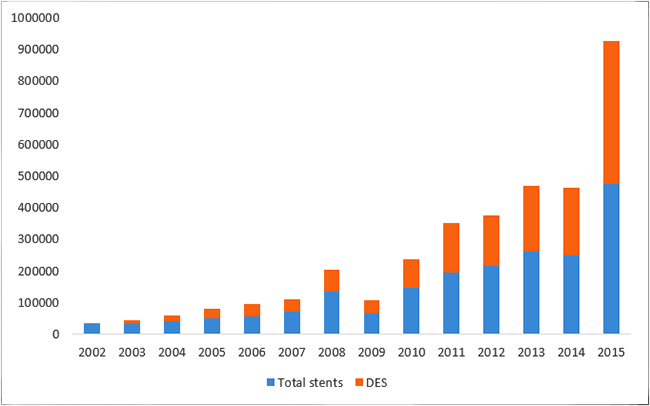
Figure 2: Sources of financing for coronary procedures (2014)[xiii]
The country’s hospital associations, meanwhile, contend that their profit margins are “justified” as they are required to invest in capital assets to ensure they are able to run their operations and provide services to their patients. The NPPA, however, argues that private hospitals do not provide any value addition and thus must cease the practice of seeking top-up margins.
Analysis and Recommendations
The NPPA order has trained the spotlight on the debate about the influence of business on healthcare, and the ethical arguments about healthcare financing in India. The questions involved in this debate include, among others: (a) the role of price controls in controlling costs in health care; (b) the role and interaction of economic regulations and related market forces in managing healthcare costs; (c) the importance of costing systems and outcomes-based measurements in managing healthcare costs; and (d) the contribution of evidence-based policymaking in managing healthcare costs.
Role of price controls in controlling healthcare costs
Many countries are, like India, grappling with a complex set of economic and ethical challenges in healthcare financing. The introduction of penalties, fines, and price caps have become common policy tools to address some of these dilemmas. The assumption is that creating such deterrence around decisions will moderate the behaviours of various players. However, keeping the rest of the eco-system unchanged around these decisions, such interventions lead to actions and decisions that may not be enduring. The NPPA order raises questions on the efficacy of price-caps and similar such strategies.
Any manufacturer or institution or person that fails to comply with the ceiling-price order of the NPPA shall be held liable to deposit the overcharged amount, along with interest thereon, to NPPA, under the provisions of the Drugs (Prices Control) Order, 2013 read with Essential Commodities Act, 1955. The ceiling price—fixed as specified in the order—shall be maintained for a period of one year from the date of NPPA’s notification unless revised by another gazette announcement. It is debatable, however, how these fines will affect the behaviour of manufacturers and sellers of cardiac stents.
Modulating the behaviour of key agents in an economic system in a desired direction has been the subject of various analyses. The fundamental hypothesis is that when regulators impose penalties or negative consequences on a particular behaviour, there would be an immediate effect on such behaviour and actors will strictly follow the pronouncement. In fact, the observation of the actual behaviour of key agents suggests that imposing adverse consequences such as penalties or limiting their behaviour may not last long and could only have a temporary impact. There is an argument that putting certain punishments with weak mechanisms to administer the undesired behaviour may not deter future behaviour.
Role of economic regulations in managing healthcare costs
Any analysis of the implications of economic regulations including price controls will need a detailed and complete specification of the market forces. In the medical market, there are demand and supply forces at work, information asymmetry prevails, and various stakeholders are involved in the making of a decision. In the marketplace, after all, a price-cap will simply be a new price. The severity of punishment, which creates the fear factor, would be crucial; besides the fear factor, to ensure its effectiveness, each intervention needs an ecosystem to establish its enduring impact, as there are several drivers at work. The promulgated regulation is associated more with profiteering behaviour than with creating a stimulus for ethical practices.
The introduction of price-caps changes the perception of people regarding the environment in which they operate. The penalty imposed in the order may alter the perception of patients differently. The way the information about the stents has been played and the tag that higher priced stents are “better”, patients’ perception may suggest that what is being provided is justified. The social interaction of provider-patient and the norms surrounding their relations may have limitations and may not be effective in negotiating the desired behaviour. On the provider side, the hospitals were charging for the cost of operation in a particular way, and in the future, they will perform that activity in the same manner but charge differently by unbundling the services. For providers, the deviation has a cost related to dented image and loss of goodwill. Based on its probability of occurrence, this cost will form part of risk management strategies of these providers, like many other risks.
The price-cap does not address the fundamental problem: that of defining the outcome and creating a stimulus for appropriate pathways and protocols. Because these are loosely defined, the actual behaviour can be moulded and justified in any manner. There is no guilt or shame that can be attached to the act of buying a service that is not required. The buying behaviour happens at will. Since the different parameters of the decision have been left unchanged, its effectiveness, therefore, will be debatable. The non-compliance penalty—which is only one parameter in the entire process—affects the decision problem of the single agent, which is, in this case, the hospital. And just to reduce their preferred level of deviation, one would be free to change the other parameters of the game. The overall result might not be what one had anticipated.
Role of costing systems and outcomes-based measurements in managing healthcare costs
Within the overall system, the hospital market has its challenges, and it is important to understand the characteristics of this market as a backdrop to the debate on the cap on pricing. One variable is contestability: where new firms are able to enter and leave a market with ease. Contestable markets have no exit/entry barriers. If in that market, prices increase way beyond the average price level (and starts generating excess profits), potential rivals will enter the market to exploit the situation. The existing players will respond by bringing down the prices appropriate to yield normal profits. The perfect contestability will ensure that even a single player can exhibit competitive behaviour.
Hospital care markets are not perfectly contestable as there are huge sunk costs creating barriers for exit and entry. A compounding factor is that the decision to use a particular input is made by the doctor and not by the patient, leading to a situation of information asymmetry between doctors and patients. Often this situation leads to induced demand problems. Many unpredictable implications and unethical behaviour on the part of hospitals may be traced to these characteristics. For example, hospital practices are replete with assertions that fee-for-service increases discretion and unnecessary clinical interventions. Given the contestability issues, even when having a large number of players, competition will not necessarily lead to efficiency and good practice. In private healthcare settings, in particular, the ability to pay takes precedence over the need, and hospital pricing policies give dominance to business interests over healthcare. India has yet to make it mandatory that doctors’ prescriptions carry the drugs’ generic names, rather than (more expensive) brands which carry no significant benefit to patient outcomes. Given the dynamics of market forces in such situations, the response from regulators is inevitable. One of the typical responses from the regulatory toolbox is following the dictum of “illegalisation of dubious pricing” policies of providers. Indeed, the efforts towards improving the contestability of medical care markets through healthcare financing policies remain inadequate.
Further, the country’s current health data are inadequate for making informed choices. India’s costing systems are not good enough to help understand the relationship between inputs, activities/processes, and outcomes, in treatment pathways. There is no cost data available on such pathways. When hospital associations make a point that cardiac stents need a delivery mechanism to be implanted within the body and that the delivery mechanism and its ecosystem require capital investments, the question remains – Why should these costs be clubbed with the cost of stents? The detailed activity-based costing of hospitals may be a way to sort these issues, and the current situation suggests that stakeholders do not have enough information on the costs of intervention. Rather, currently available cost information is related to functions such as salary, drugs, and consumables, among others. It is not possible to find the cost, for example, of an episode of hospitalisation.
The argument that stent prices are in fact within a bundled price of procedure and components is self-defeating and shows that highly reputed hospitals do not have appropriate costing systems in place. It is time for these institutions to invest in the basic infrastructure of financial management and understand their costs and present their arguments cogently. In recent times, most of the private and corporate hospitals have invested in hospital management information systems (HMIS) to manage patient care, finance, billing, medical records, and insurance clients. It is surprising that despite such improvements in information-technology infrastructure, these hospitals continue to fail in implementing data collection mechanisms for calculating costs of treatment.
Some have argued that insurance companies pay for these procedures and they are the ones that routinely produce price data. It is important to understand that the price data produced by insurance companies are negotiated prices and may have little bearing on real costs. The insurance price data are many times bundled prices, and one does not know the cost of various components of the service. Another challenge is that of not knowing the real outcome of various procedures, and there is no effort in place to capture this data. Often the process has become a proxy for outcomes and the kind of stents used. This paper contends that this is not the correct approach.
The outcomes measurements should be based on the enhancement in duration and quality of patient’s life or methods propagated on outcome measurement in a medical setting.[xiv] In private hospitals, there are huge gaps in capturing this information. Even if one captures some basic information such as readmission rates, it measures an important component of quality. The other point which also needs to be confronted is the high variation in costs and clinical activities across facilities in the country. Those working in the health sector recognise this fact, and there is little consensus on what is the best practice. In many situations, doctors do have useful insights into the input-outcome relationships, particularly what is desirable and what is not; however, they tend to ignore such knowledge because of perverse incentives. Doctors in many hospitals work based on “revenue target” approach, due to organisational pressures or third-party paying system, or a “profit motive” of the corporate/private hospitals, thus creating situations of moral hazard. Hospitals also operate in highly fragmented markets and the same is true with their procurement side. This fragmentation increases costs, as one does not get an advantage of the economies of scale.
Contribution of evidence-based policymaking in managing healthcare costs
The Indian Constitution does not accord the status of rights for access to health and healthcare.[xv] To demand access to healthcare, citizens have had to resort to judicial overreach—such as the use of Article 21 of the Constitution or various Directive Principles or references made to International Covenants.[xvi] One way to avoid incidents of judicial overreach would be to use an evidence-based approach to policymaking in India. The price ceiling on cardiac stent prices is an apt case. The NPPA ruling came after the consistent efforts of public-interest litigation over a couple of years. India would do well to establish a Medical Technology Assessment Board (MTAB), similar to that of National Institute of Health and Clinical Excellence (NICE) in the UK or Health Intervention and Technology Assessment Program (HITAP) in Thailand—so that each new drug, medical device, or technology could be subjected to a detailed assessment of clinical effectiveness, safety, and cost-effectiveness. This could help moderate the judicial overreach that has been the resort of citizens seeking access to essential healthcare.
Conclusion
About 70 percent of the INR 65,000 crore medical device market in India is dependent on imports. At present, 99 percent of all medical devices are not regulated by the government, in terms of location, utilisation, and maintenance; only about 20 devices—including cardiac stents—have been notified under the Drugs and Cosmetics Act, 1940. The proposal to establish a Medical Technology Assessment Board (MTAB) by the Department of Health Research (DHR), Government of India, was first announced in 2012, but is yet to become a reality. The Board, once established, will recommend technologies in public health (including drugs, devices, and methods of treatment) after evaluating them on their efficacy, appropriateness and cost effectiveness. In the long run, the MTAB is expected to be part of the overall regulatory structure being established in the DHR. There is an immediate urgency for the establishment of the MTAB for ensuring an evidence-based policy process rather than ‘judicial activism’ in order to improve access to cost-effective healthcare in the country.
In the last few years, there has been a greater advent of health insurance companies towards financing of healthcare. With increasing treatment costs, hospitals are being forced to provide quality services at competitive prices. This makes it necessary for hospitals to have a system for cost control, and this cost reduction without regard for outcomes leads to ‘false savings’ and is, ultimately, self-defeating. Rather, the hospitals should use the HMIS to ascertain the costs of various services after careful analysis of past utilisation data, and utilisation of capacities. The HMIS will need to be enabled to identify costs and using an allocation criteria, allocate costs, both direct and indirect, to both revenue and non-revenue centers using appropriate drivers after adequate analysis of each cost element. These efforts could provide inputs for pricing of services and surgeries, and information to the hospitals on the cost and revenue per unit of service, thus providing information on revenues, and expenses at the service level. Further, there is a need to create a system for providing high value to patients—defined as the health outcomes per rupee, rather than cost-cutting or cost-containment exercises without regard for outcomes.
Some of the players in the cardiac stent industry in the country include foreign companies such as Abbott Vascular, Boston Scientific, and Medtronic, whose R&D budgets have brought in innovations in the materials and types of cardiac stents. With the growing burden of NCDs in India, there is a need to provide accessible and quality healthcare, and access to medical devices play a critical role in managing NCDs. Given the fact that currently the market for medical devices is poorly regulated, and there is no formal system to monitor quality of care, price-control could have a negative impact in terms of entry of inferior-quality and outdated products in the market. The inclusion of the stents in the NLEM and the subsequent price-capping should be seen in light of whether such decision meets the fundamental requirements of access to innovation, quality, and patient safety. In fact, after the announcement on price-caps was made, there have been instances of hospitals increasing their procedural costs and costs for angioplasty packages.[xvii] Even though the prices of cardiac stents have fallen in the last few years, those of angioplasties have remained constant, as per the report, ‘Medical Devices in India- Accessibility, Quality and Pricing’.[xviii] It is imperative that while making such policy decisions, the government adopt a consultative approach and involve all stakeholders—clinicians, stent manufactures, patient groups, lawyers, and researchers—this ensures the creation of a stimulus for appropriate pathways and protocols.
India’s health system is facing various crises that must be resolved if the country is to improve the overall health of its citizens. Without enabling economic regulations for various public and private players to operate, transparent costing and outcomes-based measurement systems in healthcare delivery, and evidence-based healthcare policymaking, access to healthcare will remain poor and the need for ‘judicial activism’ will continue to prevail.
This paper makes the case for the establishment of an evidence-based health policy process through the setting up of a Medical Technology Assessment Board (MTAB), as well as comprehensive systems for costing of services and procedures and outcomes measurement in hospitals to manage healthcare costs and develop and implement healthcare financing policy measures to address the contestability in the healthcare markets. Indeed, the first crisis is always that of the absence of a vision. The price-cap on cardiac stents is one such example of this lack of ability to seek long-term solutions, and India’s policymakers must learn its lessons.
About the Authors
Ramesh Bhat is former Professor at IIM Ahmedabad, and President of the Indian Health Economics and Policy Association.
Denny John is Evidence Synthesis Specialist at Campbell Collaboration, New Delhi.
Endnotes
[i] Dorairaj, Prabhakaran, Panniyammakal, Jeemon and Roy, Ambuj. Cardiovascular disease in India: Current epidemiology and future directions. Circulation 133 (2016): 1605-1620
[ii] Percutaneous Coronary Intervention (PCI) is a non-surgical procedure that uses a catheter (a thin flexible tube) to place a small structure called a stent to open up blood vessels in the heart that have been narrowed by plaque buildup, a condition known as atherosclerosis.
[iii] A catheterisation laboratory (or cath-lab) is where tests and procedures including ablation, angiogram, angioplasty and implantation of pacemakers / ICDs are carried out. A cath lab is staffed by a team of different specialists, usually led by a cardiologist. A cath lab should not be confused with an operating theatre, where surgeries such as a heart bypass operation, under a general anaesthetic, are conducted.
[iv] 2010-2013 data from presentation made by Dr. Praveen Chandra, Chariman, National Intervention Council, Interventional Council of India Midterm Annual Meeting 2014- Kochi available here: https://www.slideshare.net/saketsinghi/nic-2013-registry-coronary-data-presentation-dr-praveen-chandra; 2014-15 data from newspaper reports available here: http://health.economictimes.indiatimes.com/news/industry/2014-nic-registry-reveals-68-rise-in-patients-requiring-acute-coronary-interventions/46850631
[v] Published in Part II, Section 3, Sub Section (ii) of the Gazette of India Extraordinary, 13th February 2017. Government of India, Ministry of Chemicals and Fertilizers, Department of Pharmaceuticals, National Pharmaceuticals Pricing Authority, New Delhi
[vi] Rhythma Kaul, ‘Lawyer with a heart: Birendra Sangwan’s fight to cap price of coronary stents’, Hindustan Times, Feb 20, 2017.
[vii] Rema Nagarajan, ‘Profit on stents ranges from 270% to 1000%’, Times News Network, Jan 17, 2017.
[viii] NH Political Bureau, ‘A lawyer calls the Prime Minister’s bluff on cardiac stents’, National Herald, Feb 23, 2017.
[ix] K.H. Bønaa, J. Mannsverk, R. Wiseth, L. Aaberge, Y. Myreng, O. Nygård, D.W. Nilsen, N.-E. Kløw, M. Uchto, T. Trovik, B. Bendz, S. Stavnes, R. Bjørnerheim, A.-I. Larsen, M. Slette, T. Steigen, O.J. Jakobsen, Ø. Bleie, Fossum, T.A. Hanssen, Ø. Dahl‑Eriksen, I. Njølstad, K. Rasmussen, T. Wilsgaard, and J.E. Nordrehaug, for the NORSTENT Investigators. Drug eluting stents or bare metal stents for coronary artery disease.
[x] Dianna C Carrillo Gomez, Maria C Ortiz Sierra, Magda C Cepeda Gil, Cesar A. Guevera Cuellar. Cost effectiveness of drug eluting stents versus bare metal sents in coronary artery disease: A systematic literature review. Rev Argent Caridiol 80 (2012); 366-375
[xi] J. P. Tripathy,B. M. Prasad, H. D. Shewade, A. M. V. Kumar, R. Zachariah, S. Chadha, J. Tonsing, and A. D. Harries. Cost of hospitalisation for non-communicable diseases in India: are we pro-poor? Tropical Medicine and International Health 21 (2016) 8: 1019-1028
[xii] Ramakrishnan S, Mishra S, Chakraborty R, Chandra SK, and Mardikar (2013). The report on the Indian coronary intervention data for the year 2011 – National Interventional Council. Indian Heart Journal 65 (2013) 5: 518–521. ; Cardiovascular diseases in India, Max Neeman Contract Research. http://www.neeman-medical.com/sites/default/files/files/Cardiovascular%20Diseases%20in%20India_0.pdf
[xiii] http://health.economictimes.indiatimes.com/news/industry/2014-nic-registry-reveals-68-rise-in-patients-requiring-acute-coronary-interventions/46850631
[xiv] Michael Porter, S Larsson, and Lee Thomas.Standardizing Patient Outcomes Measurement. N Engl J Med 374 (2016) : 504-506. doi: 10.1056/NEJMp1511701
[xv] M Desai and K Mahabal. Desai, M & Mahabal, KM (2007). Health care case law in India: A reader. CEHAT & ICHRL (2007). Mumbai: India
[xvi] Nariman SF, ‘Judicial overreach: It’s the order of the day’. Hindustan Times (2017), Aug 20
[xvii] Snigdha Basu, ‘Price cap on stents hailed by all, but serious concerns remain’, NDTV Health, Feb 22, 2017
[xviii] IMS Health, Medical Devices in India—Accessibility, Quality and Pricing, 2016
The views expressed above belong to the author(s). ORF research and analyses now available on Telegram! Click here to access our curated content — blogs, longforms and interviews.

 PDF Download
PDF Download





 PREV
PREV

