 human alphaherpesvirus 3
human alphaherpesvirus 3
Introduction
The largest known
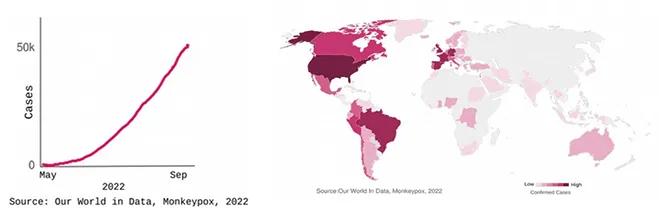
outbreak of the Monkeypox virus is currently ongoing, with over 53,000 cases and 15 deaths reported between 13 May 2022–05 September 2022. The World Health Organization (WHO) declared monkeypox a
Public Health Emergency of International Concern in July 2022. Cases have been identified in
100 countries, many of which are witnessing an outbreak for the first time.
India has reported 11 cases and 1 death as of 5 September 2022. Even as the average number of reported daily cases is declining, the global numbers continue to climb. In the present situation, testing is limited (accessibility of tests as well as willingness to get tested), vaccines exist but are unlikely to be available promptly, and medicines such as antivirals are yet to be approved and are not widely available.
In India, the VRDL network (Virus Research and Diagnostic Laboratories) is carrying out molecular testing for monkeypox, after which the samples are sent to the National Institute of Virology (NIV) in Pune for confirmation. Commercial diagnostic kits have also been developed and are available in the country.
Transmission and treatment
Monkeypox is a largely self-limiting disease and spreads mostly by close intimate contact with an infected individual or animal. It is known to infect both humans and animals—
human/animal to animal and animal to human transmission have been reported. Infected people can have a range of
symptoms such as fever, aches, and tiredness, before developing painful rashes. The number, pattern, and location of these rashes can vary. In the current outbreak, the majority of patients have exhibited rashes, with some never having a fever. These can appear on different parts of the body including the hands, face, feet, body, mouth, and on or around the anus and genitals. It takes two to four– weeks to recover though people remain infectious till the dry scabs fall off.
Monkeypox is a largely self-limiting disease and spreads mostly by close intimate contact with an infected individual or animal.
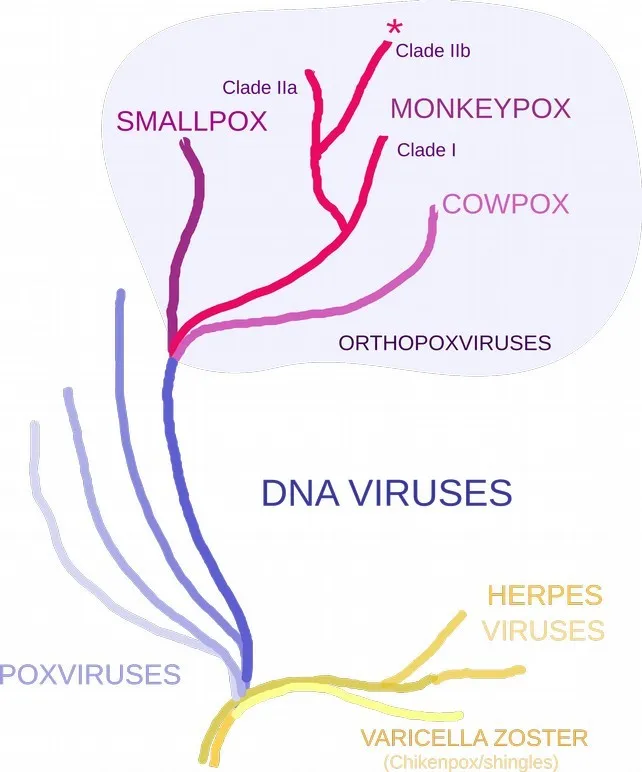
Monkeypox is a double-stranded DNA virus of the poxvirus family, with two clades—
Clade I and Clade II. These clades were known to circulate in different regions in Central Africa and West Africa, respectively. Historical outbreak data suggests that Clade I is more transmissible and is more severe than Clade II. Genomes from the current outbreak belong to the subclade IIb.
For many decades now, monkeypox has been considered a potential biological threat, and vaccines and antivirals have been in development, led by initiatives in the United States. Monkeypox is related to the smallpox virus (variola virus) but is less transmissible and causes less severe disease than the latter. During the
eradication efforts for smallpox in the 1970s, the smallpox vaccine was found to prevent the related illnesses of
monkeypox (85 percent from observational studies) and cowpox. It is likely that people who were vaccinated against smallpox (before its eradication in 1980) will have some protection from monkeypox. However, the level of cross-protection it might provide remains unclear. Monkeypox is not related to the varicella-zoster virus (
human alphaherpesvirus 3) that causes the disease varicella/chickenpox/shingles, even though the appearance of rashes and other symptoms can be similar. Both are DNA viruses but too distant from each other in the tree of life to offer cross-protection.
 Imvanex
Imvanex (manufactured by Bavarian Nordic, called Jynneos in the US), a third-generation smallpox vaccine, which has lesser side effects has been approved for use against monkeypox under exceptional circumstances as some information about the vaccine is only available from non-human studies due to the rare nature of the disease. This is a vaccine of choice in the current outbreak given its relatively favourable
safety profile compared to the older generation of smallpox vaccines.
During the eradication efforts for smallpox in the 1970s, the smallpox vaccine was found to prevent the related illnesses of monkeypox (85 percent from observational studies) and cowpox.
In terms of global vaccine availability, the WHO has
stocks of third-generation smallpox vaccines in Geneva and the Netherlands as well as some earlier-generation smallpox vaccines. The US had the largest
stockpile of Jynneos (20 million doses) but most of these expired and were not replaced. The US has placed orders for about
2 million doses which will be available in 2022. The European Union has also placed orders for over 100,000 doses of the Imvanex vaccine
via The Health Emergency Preparedness and Response (HERA); this format is meant to secure the stock and allow distribution based on the needs of individual member states and Norway and Iceland.
However, given the nature of transmission and disease (high transmission, low mortality), and shortage of vaccines, mass vaccination is not recommended. In the US, the
CDC has recommended pre-exposure prophylaxis for laboratory workers and healthcare workers who are involved in diagnostics or research work using monkeypox or related viruses. Some other agencies in Europe have recommended vaccination for high-risk groups, especially homosexuals and healthcare workers. The US CDC and European CDC currently recommend post-exposure smallpox vaccine as a method to prevent the progression of the disease or reduce its severity.
The clinical management of monkeypox is aimed at alleviating the symptoms and preventing complications. At least two antivirals developed (FDA approved) to treat smallpox are being considered for the treatment of monkeypox.
Tecovirimat (alias TPOXX) is being considered in the Expanded Access Investigational New Drug Protocol for monkeypox by the US CDC and has been licensed for use against monkeypox by the European Medicines Agency (EMA) in 2022 based on results from animal studies. Its use has been documented in a few
case studies. The other antiviral
Brincidofovir has also been used in the treatment of monkeypox. Larger studies are currently being planned both in the US and in the
UK to evaluate these drugs further. Currently, the availability of these antivirals is limited.
The CDC has recommended pre-exposure prophylaxis for laboratory workers and healthcare workers who are involved in diagnostics or research work using monkeypox or related viruses.
Conclusion
The current outbreak deserves attention for multiple reasons
: a long incubation period for the virus, where symptoms can appear three weeks after exposure; a long period of viral shedding and therefore infectiousness (1–3 weeks); reluctance to get tested or inaccessibility of tests; atypical clinical presentation; shortage of vaccines and antivirals; and the risk of severe disease in newborns, children, and immunocompromised individuals.
The monkeypox virus DNA has recently been detected in
wastewater in multiple countries (
USA,
Netherlands,
Italy, and
France). The ability to track the amount of virus in wastewater as a surrogate of infected individuals/animals is one way to keep track of the outbreak.
The key consideration right now is an undetected local outbreak that amplifies and affects a large number of people before detection. This would make containment difficult. Both the behaviour of the infected individuals as well as changes to the properties of the virus (in comparison to historical outbreaks) will influence the course of the current outbreak.
Appropriate messaging and continued monitoring of the outbreak is, therefore, essential.
The views expressed above belong to the author(s). ORF research and analyses now available on Telegram! Click here to access our curated content — blogs, longforms and interviews.



 human alphaherpesvirus 3
human alphaherpesvirus 3


 PREV
PREV


